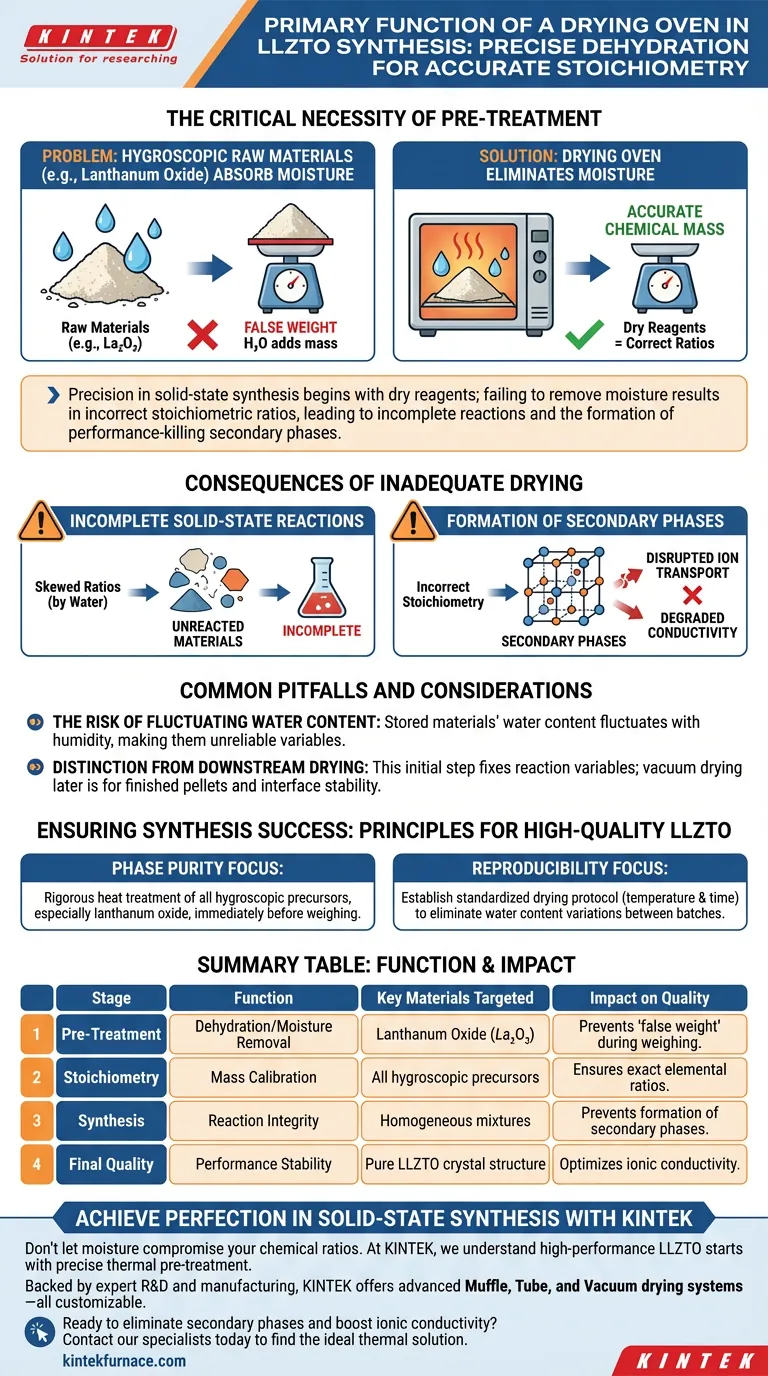
The primary function of a drying oven during the initial preparation of LLZTO solid electrolytes is to thoroughly dehydrate moisture-sensitive raw materials, most notably lanthanum oxide. By subjecting these materials to constant thermal treatment, the oven eliminates absorbed atmospheric moisture, ensuring that subsequent weighing and mixing processes are based on accurate chemical mass rather than water weight.
Precision in solid-state synthesis begins with dry reagents; failing to remove moisture results in incorrect stoichiometric ratios, leading to incomplete reactions and the formation of performance-killing secondary phases.

The Critical Necessity of Pre-Treatment
Understanding Material Sensitivity
Certain raw materials required for LLZTO synthesis, such as lanthanum oxide, are hygroscopic. This means they naturally absorb moisture from the surrounding environment.
The Problem with Absorbed Moisture
If this moisture is not removed, it adds "false weight" to the material during the weighing stage. You may believe you are measuring a specific amount of reactant, but a portion of that mass is actually water.
Ensuring Exact Stoichiometry
The drying oven acts as a calibration tool for your chemistry. By removing water, it ensures that the mass you weigh corresponds exactly to the required elemental ratios for the specific LLZTO formula.
Consequences of Inadequate Drying
Incomplete Solid-State Reactions
LLZTO synthesis relies on precise solid-state reactions between lithium, lanthanum, and zirconium sources. If the ratios are skewed by water weight, the reaction cannot proceed to completion.
Formation of Secondary Phases
When the stoichiometry is off, the chemical reaction creates byproducts rather than the pure crystal structure you intend. These "secondary phases" are impurities that disrupt ion transport and degrade the final conductivity of the electrolyte.
Common Pitfalls and Considerations
The Risk of Fluctuating Water Content
One major pitfall is assuming that raw materials stored in standard conditions remain stable. Water content fluctuates with humidity, making untreated materials an unreliable variable in your synthesis equation.
Distinction from Downstream Drying
It is important to distinguish this initial preparation step from later drying stages. While vacuum drying is often used later for finished ceramic pellets to ensure interface stability during testing, the initial drying oven stage is strictly about fixing the input variables for the chemical reaction.
Ensuring Synthesis Success
To guarantee high-quality LLZTO electrolytes, apply the following principles:
- If your primary focus is Phase Purity: Ensure all hygroscopic precursors, especially lanthanum oxide, undergo a rigorous heat treatment in the drying oven immediately before weighing.
- If your primary focus is Reproducibility: Establish a standardized drying protocol (temperature and time) to eliminate water content variations between different batches of raw materials.
Accurate synthesis is impossible without a dry baseline; the drying oven is the gatekeeper of your chemical formula.
Summary Table:
| Stage | Function | Key Materials Targeted | Impact on Quality |
|---|---|---|---|
| Pre-Treatment | Dehydration/Moisture Removal | Lanthanum Oxide ($La_2O_3$) | Prevents 'false weight' during weighing |
| Stoichiometry | Mass Calibration | All hygroscopic precursors | Ensures exact elemental ratios |
| Synthesis | Reaction Integrity | Homogeneous mixtures | Prevents formation of secondary phases |
| Final Quality | Performance Stability | Pure LLZTO crystal structure | Optimizes ionic conductivity |
Achieve Perfection in Solid-State Synthesis
Don't let moisture compromise your chemical ratios. At KINTEK, we understand that high-performance LLZTO electrolytes start with precise thermal pre-treatment. Backed by expert R&D and manufacturing, KINTEK offers advanced Muffle, Tube, and Vacuum drying systems—all customizable to meet your specific lab requirements.
Ready to eliminate secondary phases and boost ionic conductivity? Contact our specialists today to find the ideal thermal solution for your research and production needs.
Visual Guide
References
- Chaozhong Wu, Xin Xie. Reoxidation of IF Steel Caused by Cr2O3-Based Stuffing Sand and Its Optimization. DOI: 10.3390/ma18173945
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- Chairside Dental Porcelain Zirconia Sintering Furnace with Transformer for Ceramic Restorations
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
People Also Ask
- What is the importance of the constant temperature environment provided by a heating stage? Expert Lab Solutions
- What is the core function of a planetary ball mill in Bi2Te3 alloying? Drive Solid-State Reaction & Nanoscale Refinement
- What is the purpose of a laboratory vacuum chamber in sacrificial material ink prep? Ensure Structural Integrity.
- What is the wear resistance of alumina ceramics compared to manganese steel and high-chromium cast iron? Discover the Superior Choice for Abrasive Environments
- What are the advantages of using a Boron Nitride crucible? Maximize Purity and Efficiency in Laser Pyrolysis
- What is the function of the laboratory-scale condensation collection device? Optimize Multi-Stage Magnesium Separation
- What are the advantages of nickel crucibles for KOH activation? Ensure High Purity & Thermal Stability up to 700°C
- How do repeat sintering processes and specialized sintering molds address the technical challenges of manufacturing oversized flywheel rotor components? Expand Scale and Integrity



















