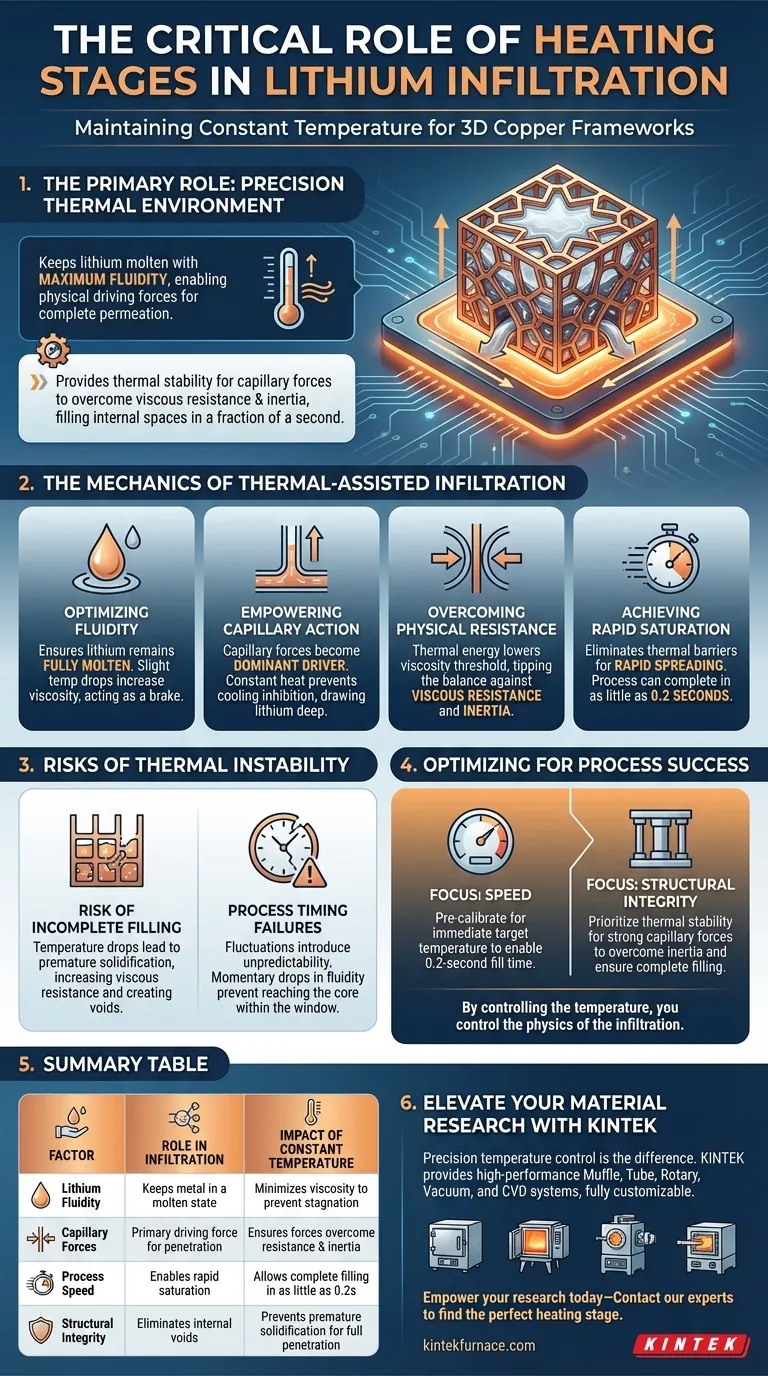
The primary role of the heating stage is to maintain a precise, stable thermal environment that keeps lithium in a molten state with maximum fluidity. This constant temperature is the enabling factor that allows physical driving forces to function correctly, ensuring the lithium permeates the complex geometry of the 3D copper framework without premature solidification.
The heating stage provides the thermal stability necessary for capillary forces to overcome viscous resistance and inertia. This allows molten lithium to completely fill the internal spaces of the framework in a fraction of a second.

The Mechanics of Thermal-Assisted Infiltration
To understand why the heating stage is non-negotiable, one must look at the physical forces at play during the infiltration process. It is a battle between driving forces and resisting forces.
Optimizing Fluidity
The immediate function of the heating stage is to ensure the lithium remains fully molten.
If the temperature drops even slightly below the optimal range, the lithium's viscosity increases. High viscosity acts as a brake on the process, making the metal sluggish and difficult to move through small pores.
Empowering Capillary Action
Under constant temperature conditions, capillary forces become the dominant driver of the process.
These forces naturally pull the liquid into the narrow channels of the copper framework. The heating stage ensures these forces are not inhibited by cooling, allowing them to draw the lithium deep into the structure.
Overcoming Physical Resistance
The infiltration process faces two main opponents: viscous resistance and inertia.
Viscous resistance tries to stop the flow of the liquid, while inertia resists the initial movement. The thermal energy provided by the heating stage lowers the viscosity threshold, tipping the balance in favor of the capillary forces so they can overpower these resistances.
Achieving Rapid Saturation
When the temperature is held constant, the speed of infiltration is drastic.
The primary reference notes that the process can be completed in as little as 0.2 seconds. This rapid spreading is only possible because the constant heat eliminates thermal barriers that would otherwise slow down the flow.
Risks of Thermal Instability
While the heating stage enables the process, understanding the consequences of thermal variation highlights its critical importance.
The Risk of Incomplete Filling
If the heating stage fails to provide a constant temperature, the lithium may cool upon contact with the copper.
This increases viscous resistance immediately. If this resistance exceeds the capillary force, the lithium will stop moving, resulting in a partially filled framework with voids that compromise the final material's performance.
Process Timing Failures
The infiltration window is extremely short.
Any fluctuation in temperature introduces unpredictability into the flow rate. In a process measured in tenths of a second, even a momentary drop in fluidity can prevent the lithium from reaching the core of the framework before the process window closes.
optimizing for Process Success
To ensure a successful lithium infiltration, you must view the heating stage not just as a heater, but as a viscosity control system.
If your primary focus is Speed: Ensure the heating stage is pre-calibrated to maintain the target temperature immediately, enabling the 0.2-second fill time.
If your primary focus is Structural Integrity: Prioritize thermal stability to guarantee that capillary forces remain strong enough to overcome inertia and completely fill all internal spaces.
By controlling the temperature, you control the physics of the infiltration.
Summary Table:
| Factor | Role in Infiltration | Impact of Constant Temperature |
|---|---|---|
| Lithium Fluidity | Keeps metal in a molten state | Minimizes viscosity to prevent flow stagnation |
| Capillary Forces | Primary driving force for penetration | Ensures forces overcome resistance and inertia |
| Process Speed | Enables rapid saturation | Allows complete filling in as little as 0.2 seconds |
| Structural Integrity | Eliminates internal voids | Prevents premature solidification for full penetration |
Elevate Your Material Research with KINTEK
Precision temperature control is the difference between a successful 0.2-second infiltration and a failed experiment. At KINTEK, we understand that your breakthroughs depend on thermal stability. Backed by expert R&D and world-class manufacturing, we provide high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique laboratory requirements.
Whether you are developing next-generation battery frameworks or advanced alloys, our heating solutions offer the reliability you need to master fluid dynamics and material saturation. Empower your research today—Contact our experts at KINTEK to find the perfect heating stage for your application.
Visual Guide
References
- Inyeong Yang, Sanha Kim. Ultrathin 3D Cu/Li Composite with Enhanced Li Utilization for High Energy Density Li‐Metal Battery Anodes. DOI: 10.1002/smll.202501629
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- Why is a high-performance vacuum pumping system necessary for industrial alloys? Ensure Purity & Peak Performance
- Why is it necessary to use alumina or ceramic crucibles during the high-temperature evaporation of magnesium? Ensure Purity and Process Integrity
- Why is a vacuum pumping system essential for DD6 alloy and ceramic shell experiments? Achieve High-Purity Results
- What are the benefits of a vacuum chamber? Achieve Unmatched Process Control and Purity
- What environmental conditions do vacuum systems and quartz tubes provide? Optimize ZnS Nanobelt Synthesis
- Why is a vacuum pump utilized in research concerning the reaction of magnesium with carbon dioxide and nitrogen? Ensure Data Integrity
- What is the function of molybdenum fixtures in high-temperature heat treatment? Ensure Perfect Diffusion Integrity
- What are the material requirements for a quartz boat in APVT? Ensure High-Purity Sb2Se3 Nanowire Growth



















