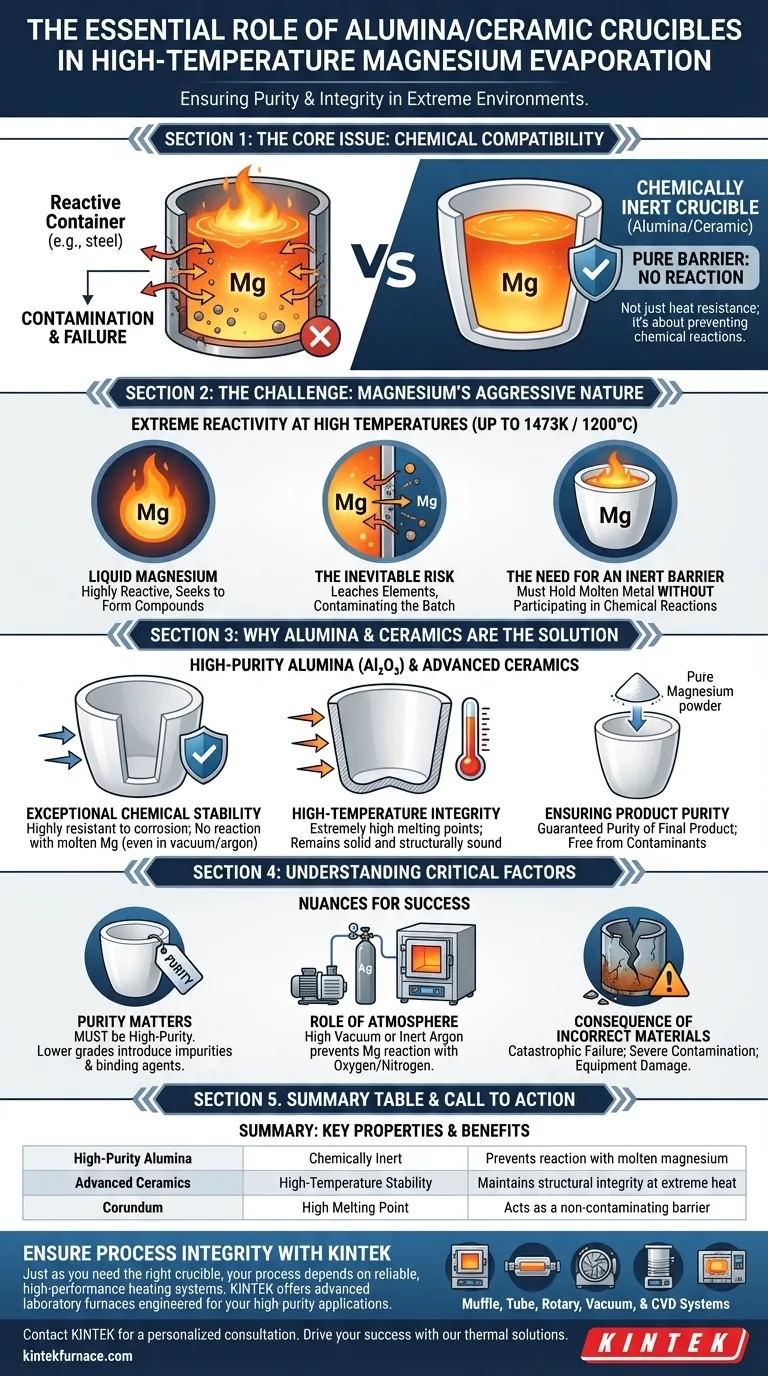
In short, alumina and ceramic crucibles are necessary because they are chemically inert and can withstand the extreme temperatures required for magnesium evaporation. At high temperatures, molten magnesium is incredibly reactive and will attack and dissolve most other materials, but high-purity alumina remains stable, acting as a clean, non-contaminating container.
The core issue is not simply heat resistance, but chemical compatibility. Choosing a crucible is a deliberate act of chemical engineering to prevent the container itself from becoming a source of contamination, thereby ensuring the purity of the final magnesium product.

The Challenge: Magnesium's Aggressive Nature at High Temperatures
To understand the specific need for alumina, we must first appreciate the hostile environment created during magnesium evaporation. This is a process of extremes, where material selection is critical.
Extreme Chemical Reactivity
Liquid magnesium is not a passive substance. As it approaches its evaporation point at high temperatures (processes can reach up to 1473K or 1200°C), its chemical reactivity skyrockets. It actively seeks to form compounds with other elements.
The Inevitable Risk of Contamination
If the crucible material is not stable, the molten magnesium will react with it. This reaction leaches elements from the container directly into the magnesium, contaminating the entire batch and compromising the purity of the final product.
The Need for an Inert Barrier
The crucible's primary job is to function as a completely inert barrier. It must hold the molten metal without participating in any chemical reactions. This ensures that the only substance being evaporated is the magnesium itself.
Why Alumina and Ceramics are the Solution
High-purity alumina (aluminum oxide, Al₂O₃) and similar advanced ceramics are specifically chosen because their properties directly counter the challenges posed by molten magnesium.
Exceptional Chemical Stability
The defining characteristic of these materials is their chemical stability. They are highly resistant to corrosion and do not react with molten magnesium, even under high-temperature and high-vacuum or argon atmosphere conditions.
High-Temperature Integrity
In addition to being chemically inert, these ceramics possess extremely high melting points. They remain solid and structurally sound well past the temperatures required to evaporate magnesium, preventing any risk of the container melting or deforming.
Ensuring Product Purity
The direct result of using an inert, stable crucible is the guaranteed purity of the final product. When the magnesium evaporates and is subsequently condensed into a powder, it is free from contaminants that would have otherwise been introduced by a reactive container.
Understanding the Critical Factors
Simply choosing "ceramic" is not enough. The success of the process depends on understanding the nuances of the material and environment.
Purity of the Crucible Matters
The references specify high-purity alumina or corundum. This is a critical detail. A lower-grade ceramic may contain impurities or binding agents that could themselves leach into the molten magnesium, defeating the purpose of using a ceramic crucible in the first place.
The Role of Atmosphere
The process is typically conducted under a high vacuum or an inert argon atmosphere. This works in tandem with the inert crucible to prevent contamination. An inert atmosphere prevents the highly reactive molten magnesium from reacting with oxygen or nitrogen in the air.
The Consequence of Incorrect Materials
Using a container made from a reactive material, such as steel or a standard glass, would be catastrophic. The molten magnesium would rapidly degrade the crucible, leading to severe contamination of the magnesium and likely causing a complete failure of the experiment or production run.
Making the Right Choice for Your Process
Your choice of container is a foundational decision that dictates the quality of your results.
- If your primary focus is maximizing purity: You must use the highest-grade alumina or corundum crucible available to minimize any potential for trace contamination.
- If your primary focus is experimental repeatability: Consistently use the same type and grade of ceramic crucible to ensure the container is not an uncontrolled variable in your results.
- If your primary focus is avoiding catastrophic failure: Never substitute with materials not explicitly rated for contact with molten magnesium, as this will lead to certain contamination and potential equipment damage.
Ultimately, selecting the correct crucible is the first line of defense in safeguarding the integrity of your material and the success of your high-temperature process.
Summary Table:
| Crucible Material | Key Property | Benefit for Magnesium Evaporation |
|---|---|---|
| High-Purity Alumina | Chemically Inert | Prevents reaction with molten magnesium |
| Advanced Ceramics | High-Temperature Stability | Maintains structural integrity at extreme heat |
| Corundum | High Melting Point | Acts as a non-contaminating barrier |
Ensure the Integrity of Your High-Temperature Processes with KINTEK
Selecting the right crucible is critical for the success and purity of your high-temperature applications, like magnesium evaporation. Just as this article highlights the necessity of chemically inert, high-purity alumina, your entire thermal process depends on reliable, high-performance equipment.
KINTEK's advanced laboratory furnaces and heating systems are engineered to meet these exacting demands. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable for your unique needs. Our solutions provide the precise temperature control and stable environment required to maximize the effectiveness of your high-purity crucibles and ensure uncontaminated results.
Ready to enhance your process reliability and product purity? Let our experts help you select the perfect system.
Contact KINTEL today for a personalized consultation and discover how our thermal solutions can drive your success.
Visual Guide
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What types of production processes benefit from the thermal uniformity of tube furnaces? Enhance Precision in Material Processing
- Why is uniform heating important in tubular furnaces? Ensure Process Reliability and Predictable Results
- How do roller kilns and tube furnaces differ in their use of Alumina ceramic tubes? Compare Transport vs. Containment
- What industries benefit from the use of tube furnaces? Unlock Precision in Semiconductor and Battery Tech
- What is flash vacuum pyrolysis and how is a tube furnace utilized in this process? Unlock High-Temp Chemical Reactions



















