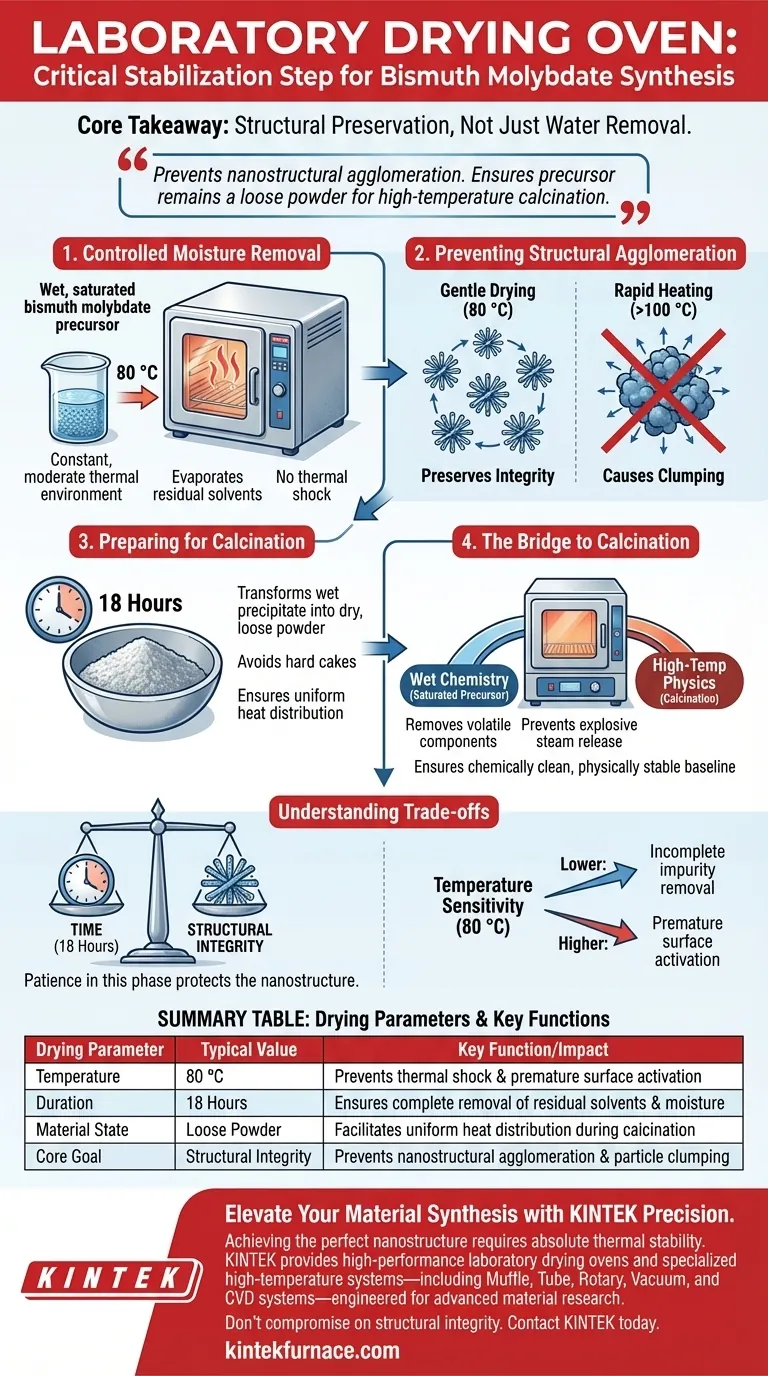
A laboratory drying oven serves as a critical stabilization step in the synthesis of solid bismuth molybdate. Its primary function is to provide a constant, moderate thermal environment—typically around 80 °C for 18 hours—to gently remove residual liquid impurities and moisture from the washed precursor material.
Core Takeaway: The drying phase is not merely about water removal; it is a structural preservation technique. By using low, sustained heat, the oven prevents the nanostructural agglomeration that occurs during rapid temperature spikes, ensuring the precursor remains a loose powder ready for high-temperature calcination.

The Mechanics of Precursor Stabilization
Controlled Moisture Removal
After the initial washing of solid bismuth molybdate, the material is saturated with residual solvents and water.
The drying oven creates a stable thermal environment, usually maintained at 80 °C. This moderate temperature is sufficient to evaporate liquids without subjecting the material to thermal shock.
Preventing Structural Agglomeration
One of the primary risks during synthesis is the clumping, or agglomeration, of nanostructures.
If the damp material were exposed to rapid temperature increases immediately, surface tension and uneven drying rates would force particles to bind tightly together. The drying oven’s gentle heating profile mitigates this, preserving the individual integrity of the nanostructures.
Preparing for High-Temperature Calcination
Ensuring a "Loose" State
The goal of the drying process is to transform the wet precipitate into a dry, loose powder.
By slowly eliminating moisture over a period of 18 hours, the material avoids forming hard cakes or dense aggregates. This "loose state" is essential for uniform heat distribution in the next stage of processing.
The Bridge to Calcination
The drying oven acts as a necessary bridge between wet chemistry and high-temperature physics.
It removes volatile components that could cause explosive steam release or structural collapse during the subsequent calcination process. This ensures the baseline material is chemically clean and physically stable before it undergoes intense heat treatment.
Understanding the Trade-offs
Time vs. Structural Integrity
The 18-hour duration is a significant investment of time, but it is a necessary trade-off for quality.
Rushing this process by increasing the temperature to speed up drying (e.g., above 100°C) risks triggering the very agglomeration you are trying to avoid. Patience in this phase protects the nanostructure.
Temperature Sensitivity
While effective for moisture, the 80 °C setpoint is specific to preserving this particular precursor.
Deviating significantly lower may fail to remove all impurities, while higher temperatures could prematurely activate surface changes or densify the powder, rendering the final catalyst less effective.
Making the Right Choice for Your Goal
To ensure the highest quality bismuth molybdate material, align your drying protocol with your specific objectives:
- If your primary focus is Nanostructure Preservation: Adhere strictly to the gentle 80 °C limit to prevent particle clumping and preserve surface area.
- If your primary focus is Process Consistency: Maintain the full 18-hour duration to guarantee that every batch enters the calcination phase with identical moisture content.
The drying oven is not just a dehydrator; it is the tool that defines the physical texture and uniformity of your final catalyst.
Summary Table:
| Drying Parameter | Typical Value | Key Function/Impact |
|---|---|---|
| Temperature | 80 °C | Prevents thermal shock and premature surface activation |
| Duration | 18 Hours | Ensures complete removal of residual solvents and moisture |
| Material State | Loose Powder | Facilitates uniform heat distribution during calcination |
| Core Goal | Structural Integrity | Prevents nanostructural agglomeration and particle clumping |
Elevate Your Material Synthesis with KINTEK Precision
Achieving the perfect nanostructure requires more than just heat; it requires absolute thermal stability. KINTEK provides high-performance laboratory drying ovens and specialized high-temperature systems—including Muffle, Tube, Rotary, Vacuum, and CVD systems—engineered to meet the rigorous demands of advanced material research.
Whether you are preparing bismuth molybdate catalysts or complex ceramics, our expert R&D and manufacturing teams offer customizable solutions tailored to your unique laboratory needs. Don't compromise on structural integrity. Contact KINTEK today to discover how our precision heating equipment can optimize your precursor stabilization and calcination workflows.
Visual Guide
References
- Thi Thanh Hoa Duong, Norbert Steinfeldt. Enhanced Photocatalytic Drug Degradation via Nanoscale Control of Bismuth Molybdate. DOI: 10.1021/acsanm.5c03249
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- How does a high-precision laboratory oven ensure the performance of large-scale halide perovskite catalyst plates?
- How does a sputtering system contribute to the preparation of electrodes? Enhance Bismuth Telluride Characterization
- How does high-purity argon gas affect the production of ultrafine magnesium powder in evaporation-condensation methods? Master Particle Size Control
- How does a programmable high-temperature furnace improve the control of cooling rates? Enhance Ceramic Part Integrity
- Why is thermal annealing of native substrates required for β-Ga2O3? Optimize Your Epitaxial Growth Foundation
- What is the mechanism of using TeCl4 as a gaseous transport agent? Grow High-Integrity Single Crystals with Ease
- What is the purpose of using a vacuum dryer for PU and AlN composite sheets? Enhance Thermal & Structural Integrity
- What is the role of calcination using high-temperature furnaces in the top-down synthesis of ZnO-NPs?



















