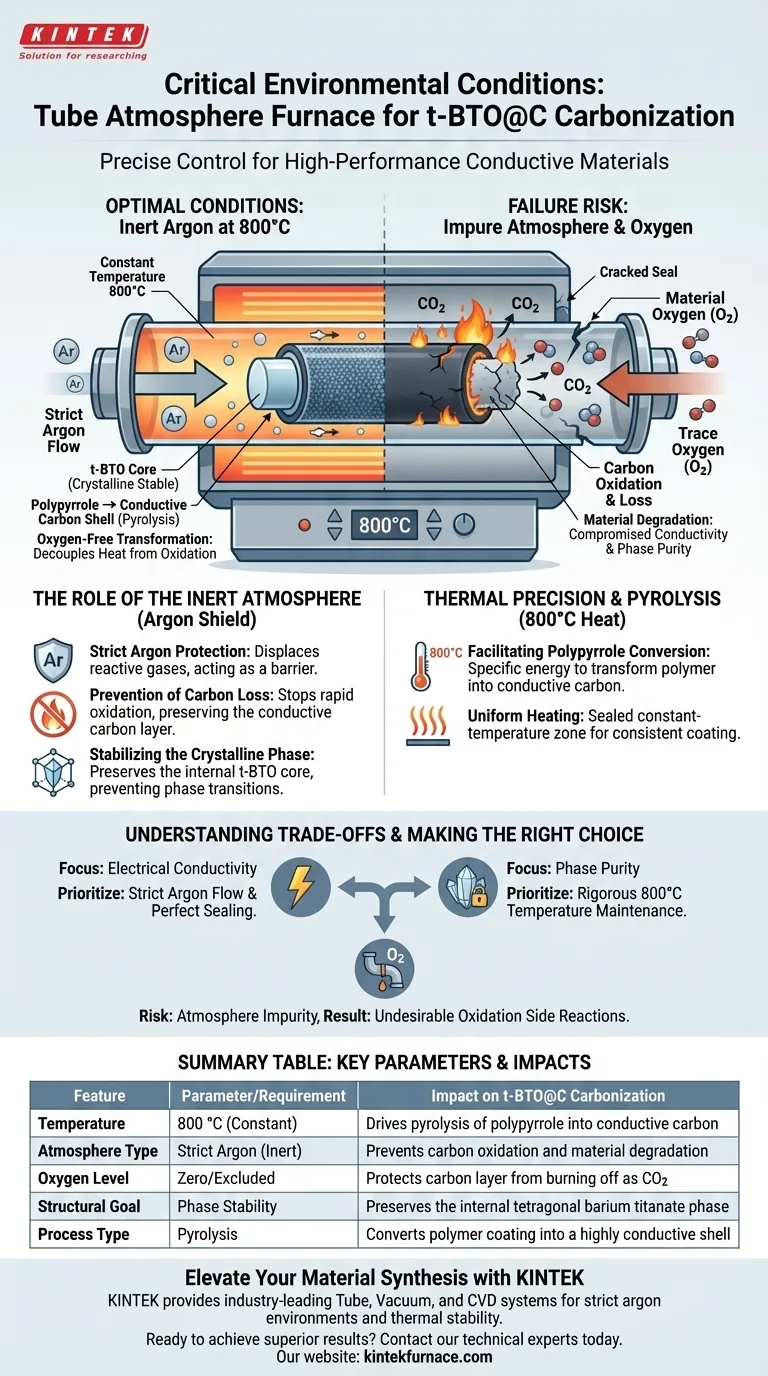
During the carbonization of t-BTO@C materials, the tube atmosphere furnace provides a precise high-temperature environment of 800 °C maintained under a strict, inert argon atmosphere. This specific combination is required to drive the pyrolysis of the polypyrrole layer into conductive carbon while totally excluding oxygen to prevent material degradation.
The core function of this environment is to decouple heat from oxidation. By sustaining an oxygen-free argon atmosphere at high temperatures, the furnace allows for the chemical transformation of the outer layer without burning away the carbon or destabilizing the internal crystalline structure.

The Role of the Inert Atmosphere
Strict Argon Protection
For t-BTO@C synthesis, the furnace must utilize argon rather than standard air.
This inert gas acts as a shield, displacing reactive gases that would otherwise interact with the sample during the heating process.
Prevention of Carbon Loss
The most critical function of this atmosphere is preventing oxidation.
At 800 °C, carbon reacts rapidly with oxygen. Without the sealed argon environment, the newly formed conductive carbon layer would burn off as carbon dioxide, leaving the material useless.
Stabilizing the Crystalline Phase
Beyond protecting the carbon, the inert environment preserves the core material.
The argon atmosphere ensures the stability of the internal t-BTO (tetragonal barium titanate) crystalline phase, preventing unwanted phase transitions that could occur in a reactive environment.
Thermal Precision and Pyrolysis
Facilitating Polypyrrole Conversion
The furnace maintains a constant temperature of 800 °C.
This specific thermal energy is required to pyrolyze the polypyrrole coating, effectively transforming the polymer into a highly conductive carbon layer.
Uniform Heating
The tube furnace design offers a sealed, constant-temperature zone.
This ensures that the carbonization process occurs uniformly across the material, resulting in a consistent conductive coating.
Understanding the Trade-offs
The Risk of Atmosphere Impurity
The process is intolerant to leaks. Even a minor failure in the sealing system or the gas supply can introduce trace oxygen.
If the atmosphere is not strictly inert, undesirable oxidation side reactions will occur immediately, compromising the fixation of the carbon layer.
Thermal Specificity
The temperature of 800 °C is a precise operational parameter for this specific material.
Deviating significantly from this temperature could result in incomplete pyrolysis (if too low) or potential thermal shock to the ceramic core (if uncontrolled), emphasizing the need for the precise control a tube furnace offers.
Making the Right Choice for Your Goal
To ensure high-quality t-BTO@C synthesis, align your furnace parameters with your specific material objectives:
- If your primary focus is Electrical Conductivity: Prioritize a strict argon flow and perfect sealing to ensure the polypyrrole layer carbonizes fully without oxidizing and vanishing.
- If your primary focus is Phase Purity: Maintain the temperature rigorously at 800 °C, as this specific heat treatment ensures the t-BTO crystalline phase remains stable within the carbon shell.
Success in this process is defined by the absolute exclusion of oxygen during the high-temperature transformation.
Summary Table:
| Feature | Parameter/Requirement | Impact on t-BTO@C Carbonization |
|---|---|---|
| Temperature | 800 °C (Constant) | Drives pyrolysis of polypyrrole into conductive carbon |
| Atmosphere Type | Strict Argon (Inert) | Prevents carbon oxidation and material degradation |
| Oxygen Level | Zero/Excluded | Protects carbon layer from burning off as CO2 |
| Structural Goal | Phase Stability | Preserves the internal tetragonal barium titanate phase |
| Process Type | Pyrolysis | Converts polymer coating into a highly conductive shell |
Elevate Your Material Synthesis with KINTEK
Precise carbonization of t-BTO@C materials demands absolute control over temperature and atmospheric purity. KINTEK provides industry-leading Tube, Vacuum, and CVD systems designed to maintain the strict argon environments and thermal stability your research requires. Backed by expert R&D and manufacturing, our lab high-temp furnaces are fully customizable to meet your unique processing needs, ensuring phase purity and optimal conductivity every time.
Ready to achieve superior results? Contact our technical experts today to find the perfect furnace solution for your laboratory.
Visual Guide
References
- Rui Li, Shi Chen. Ferroelectricity enhances ion migration in hard carbon anodes for high-performance potassium ion batteries. DOI: 10.1039/d4nr04916k
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What are the heating mechanisms used in retort furnaces? Choose the Right Heating for Your Lab or Industry
- What are the commonly used inert gases in atmosphere furnaces? Optimize Your Heat Treatment Process
- Why is a continuous argon flow necessary during the thermal treatment of graphite? Achieve 2400 °C Ultra-Deep Purification
- What safety mechanisms are included in controlled atmosphere furnaces? Essential Features for Hazard-Free Operation
- Why is it necessary to use an atmosphere furnace for MOF melt-quenching? Protect Fragile Materials from Decomposition
- How does the box type annealing atmosphere furnace improve production efficiency? Boost Throughput and Cut Costs
- Why are inert ovens important in electronics manufacturing? Prevent Oxidation and Boost Component Reliability
- How are retort furnaces utilized in laboratory environments? Unlock Precise Atmospheric Control for Advanced Research



















