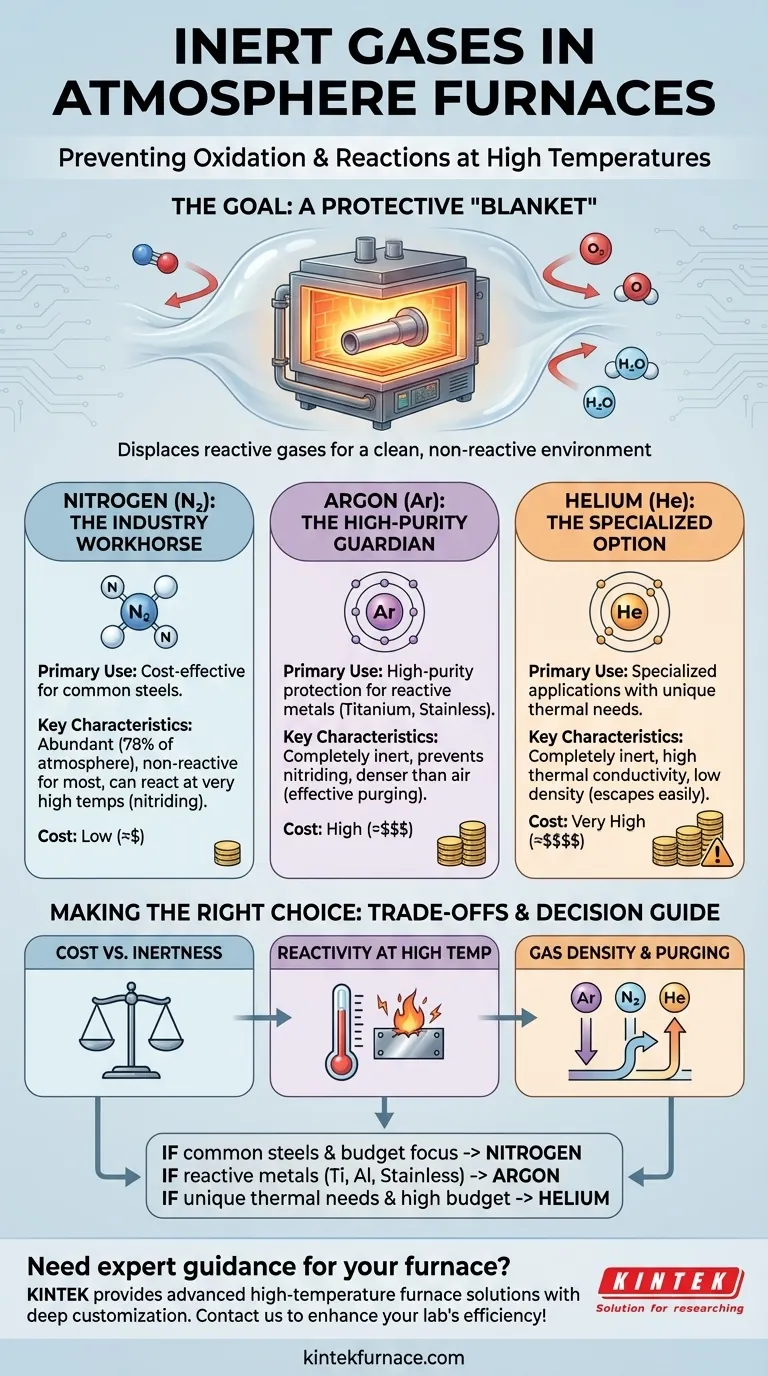
The most common inert gases used in atmosphere furnaces are nitrogen, argon, and, to a lesser extent, helium. Their primary purpose is to displace oxygen and other reactive gases, creating a protective, non-reactive environment that prevents oxidation and other unwanted chemical reactions during high-temperature processing.
Choosing the right inert gas is a critical decision based on a balance of cost, the reactivity of the material being processed, and the required level of purity. While nitrogen is the most common choice, it is not universally suitable for all materials and temperatures.

The Goal: Preventing Reactions at High Temperatures
What is an Inert Atmosphere?
At the high temperatures used in processes like annealing or brazing, many materials become highly reactive. When exposed to normal air, metals will rapidly oxidize, forming scale on their surface that degrades their properties and finish.
An inert atmosphere displaces the reactive oxygen, water vapor, and carbon dioxide in the air. By filling the furnace chamber with a non-reactive gas, the material is shielded from these harmful interactions.
The Role of Inert Gases
Inert gases like nitrogen and argon are chemically stable due to their full outer electron shells. This makes them extremely reluctant to form chemical bonds with other elements, even at high temperatures.
They act as a stable, protective "blanket" around the workpiece, ensuring that the only changes occurring are those intended by the heat treatment process itself.
Common Applications
This protective environment is essential for a wide range of industrial processes, including:
- Annealing: Softening metals to improve ductility.
- Brazing: Joining two metals using a filler material.
- Sintering: Fusing powdered materials together with heat.
- Hardening: Heat-treating metals to increase their hardness.
- Curing: Using heat to finalize the properties of a polymer or composite.
A Breakdown of Common Inert Gases
Nitrogen (N₂): The Industry Workhorse
Nitrogen is the most widely used inert gas for atmosphere furnaces, primarily because it is abundant and highly cost-effective. It constitutes about 78% of the Earth's atmosphere, making it relatively inexpensive to produce.
For the vast majority of applications, especially the heat treatment of common steels, nitrogen provides excellent protection against oxidation.
Argon (Ar): The High-Purity Guardian
Argon is significantly more inert than nitrogen. While nitrogen is non-reactive in most situations, it can react with certain highly reactive metals at elevated temperatures to form unwanted nitrides.
Argon does not share this limitation. It remains completely inert under virtually all heat treatment conditions, making it the required choice for processing sensitive materials like titanium, certain stainless steels, and other reactive alloys.
Helium (He): The Specialized Option
Helium is also completely inert, similar to argon. However, it is much less common in furnace applications due to its significantly higher cost and low natural abundance.
Its primary advantages are its low density and high thermal conductivity, which can be useful in niche applications requiring very specific heating or cooling rates. For most standard thermal processes, its cost is prohibitive.
Understanding the Trade-offs
Cost vs. Required Inertness
The most significant trade-off is between cost and performance. Nitrogen is the clear economic choice and is sufficient for a large portion of heat-treating work.
The higher cost of argon is only justified when the material demands a level of purity that nitrogen cannot provide. Using argon for a simple steel part would be unnecessarily expensive.
Reactivity at High Temperatures
This is a critical distinction. Nitrogen's "inert" nature has limits. At very high temperatures, it can react with elements like titanium, aluminum, and magnesium. This reaction, known as nitriding, can make the material brittle.
In these specific cases, argon is not just a better option—it is the only correct option to preserve the material's integrity.
Gas Density and Purging
Practical handling is also a factor. Argon is about 40% denser than air, which makes it very effective at purging a furnace chamber, as it settles and displaces lighter air upwards.
Nitrogen has a density very similar to air, requiring more careful flow management to ensure a complete purge. Helium, being much lighter than air, will rapidly escape any leaks and requires a well-sealed furnace.
Making the Right Choice for Your Process
The optimal choice depends directly on your material, process parameters, and budget.
- If your primary focus is cost-effective treatment of common steels: Nitrogen is almost always the most economical and effective choice.
- If you are working with reactive metals like titanium, aluminum, or certain grades of stainless steel: Argon is the required standard to prevent unwanted chemical reactions like nitriding.
- If your process has unique thermal requirements and a flexible budget: Helium's high thermal conductivity might offer advantages, but it is rarely the first or most practical choice.
Ultimately, selecting the correct inert atmosphere is a foundational step in achieving consistent, high-quality results in thermal processing.
Summary Table:
| Gas | Primary Use | Key Characteristics | Cost |
|---|---|---|---|
| Nitrogen | Cost-effective for common steels | Abundant, non-reactive in most cases | Low |
| Argon | High-purity protection for reactive metals | Completely inert, prevents nitriding | High |
| Helium | Specialized applications | High thermal conductivity, low density | Very High |
Need expert guidance on selecting the right inert gas for your furnace? KINTEK leverages exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures we can precisely meet your unique experimental requirements. Contact us today to enhance your lab's efficiency and achieve superior results!
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What are the primary inert gases used in vacuum furnaces? Optimize Your Heat Treatment Process
- What are the development prospects of atmosphere box furnaces in the aerospace industry? Unlock Advanced Material Processing for Aerospace Innovation
- How do argon and nitrogen protect samples in vacuum furnaces? Optimize Your Thermal Process with the Right Gas
- What are some specific applications of atmosphere furnaces in the ceramics industry? Enhance Purity and Performance
- Can box type high-temperature resistance furnaces control the atmosphere? Unlock Precision in Material Processing



















