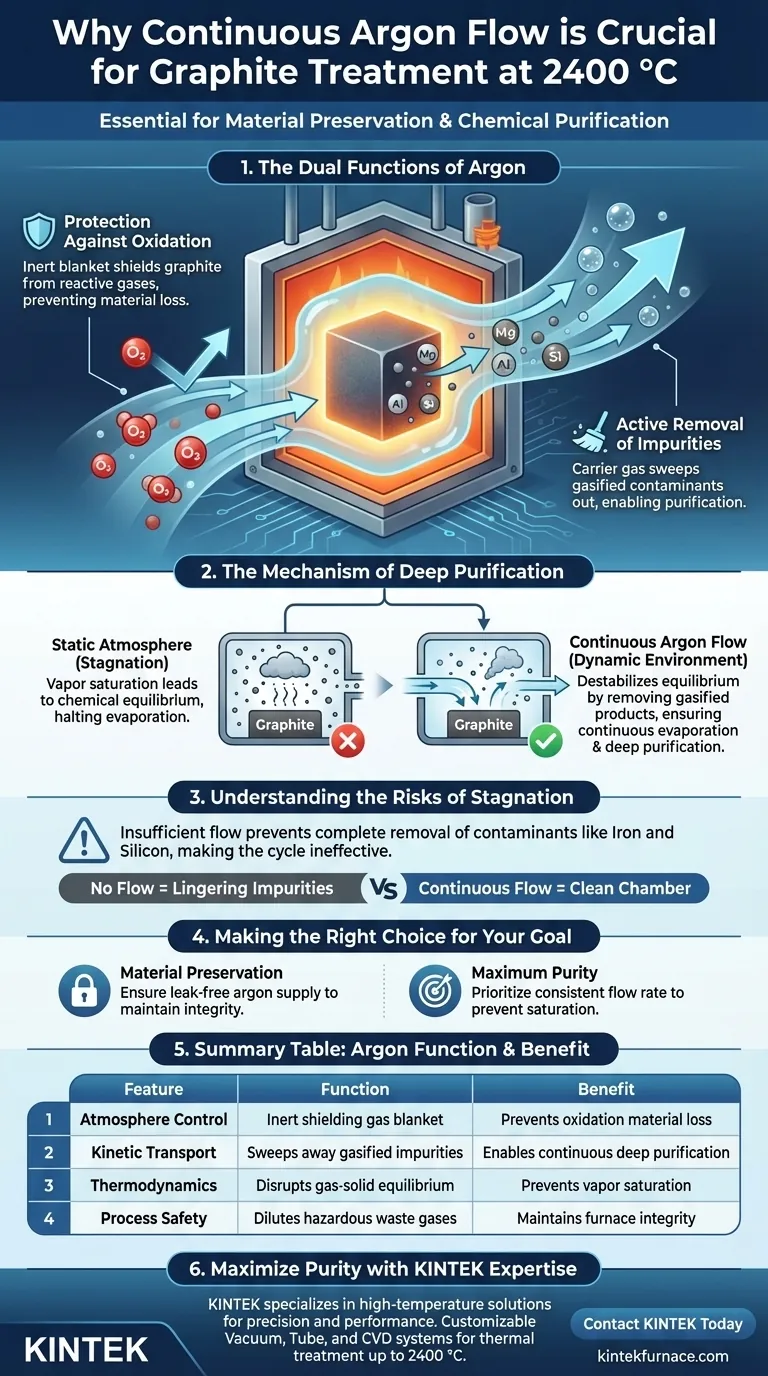
A continuous flow of argon is essential for both material preservation and chemical purification. At 2400 °C, the argon stream acts as a dual-purpose mechanism: it shields the graphite structure from oxidation while simultaneously driving the kinetic removal of vaporized contaminants.
The success of high-temperature treatment relies on disrupting chemical equilibrium. Argon flow prevents the atmosphere from becoming saturated with impurity vapors, ensuring continuous evaporation and deep purification.

The Dual Functions of Argon
Protection Against Oxidation
At extreme temperatures like 2400 °C, graphite is highly susceptible to degradation if exposed to reactive gases.
Argon serves as an inert "blanket" within the furnace chamber. This creates a protective environment that strictly prevents the oxidation and subsequent loss of the graphite material itself.
Active Removal of Impurities
The second critical function of the argon flow is its role as a carrier gas.
During treatment, metallic impurities embedded in the graphite—specifically magnesium, aluminum, iron, and silicon—are converted into gasified products. The moving argon stream physically sweeps these gasified contaminants out of the furnace chamber.
The Mechanism of Deep Purification
Disrupting Thermodynamic Equilibrium
Effective purification requires more than just high heat; it requires a dynamic environment.
In a static atmosphere, the space around the graphite would eventually become saturated with impurity vapors, establishing a gas-solid or gas-liquid equilibrium. Once this equilibrium is reached, evaporation stops, and purification halts.
Driving Continuous Evaporation
The continuous flow of argon constantly destabilizes this equilibrium.
By removing the gasified products immediately as they form, the argon flow ensures the partial pressure of impurities in the atmosphere remains low. This forces the system to continue evaporating impurities from the graphite to restore the balance, enabling deep purification.
Understanding the Risks of Stagnation
The Consequence of Insufficient Flow
It is a common misconception that temperature alone drives purification.
Without a continuous flow to carry away waste gases, impurities will linger in the furnace chamber. This stagnation prevents the complete removal of contaminants like iron and silicon, rendering the high-temperature cycle ineffective for achieving high-purity grades.
Making the Right Choice for Your Goal
To optimize your thermal treatment process, consider these operational priorities:
- If your primary focus is Material Preservation: Ensure the argon supply is completely free of oxygen leaks to maintain the integrity of the graphite mass.
- If your primary focus is Maximum Purity: Prioritize a consistent, uninterrupted flow rate to prevent vapor saturation and drive the continuous evaporation of deep-seated impurities.
The argon flow is not merely a passive shield; it is the active transport mechanism that makes deep purification physically possible.
Summary Table:
| Feature | Function of Argon at 2400 °C | Benefit to Graphite |
|---|---|---|
| Atmosphere Control | Provides an inert shielding gas blanket | Prevents material loss due to oxidation |
| Kinetic Transport | Sweeps gasified magnesium, iron, and silicon away | Enables continuous deep purification |
| Thermodynamics | Disrupts gas-solid chemical equilibrium | Prevents vapor saturation for higher purity |
| Process Safety | Dilutes and removes hazardous waste gases | Maintains furnace integrity and performance |
Maximize Your Material Purity with KINTEK Expertise
Don't let stagnant furnace atmospheres compromise your high-purity graphite production. At KINTEK, we specialize in high-temperature solutions engineered for precision and performance. Backed by expert R&D and manufacturing, we offer customizable Vacuum, Tube, and CVD systems designed to handle the rigorous demands of thermal treatment up to 2400 °C.
Whether you need optimized gas flow dynamics or a custom high-temp furnace, our team is ready to deliver specialized lab systems for your unique needs. Contact KINTEK today to speak with an expert and enhance your lab's thermal processing efficiency!
Visual Guide
References
- Anna Lähde, Jorma Jokiniemi. Effect of high temperature thermal treatment on the electrochemical performance of natural flake graphite. DOI: 10.1557/s43578-024-01282-z
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- Why is a laboratory chamber with a controlled atmosphere necessary for the laser reduction of graphene oxide (rGO)?
- What is the primary function of the vacuum atmosphere in diamond tool sintering? Prevent Oxidation & Enhance Bonding
- How does the design of a convector plate affect the thermal efficiency? Maximize Bell-Type Annealing Performance
- What are the key features of an atmosphere box furnace? Unlock Precise Heat Processing in Controlled Environments
- Why is a uniform atmosphere important in carburizing workpieces? Ensure Consistent Hardness and Prevent Failures
- What are the key features of calcining furnaces? Boost Efficiency and Quality in Material Processing
- What are the consequences of an improperly controlled furnace atmosphere? Avoid Costly Defects and Safety Hazards
- What is the purpose of the 1000 °C pre-annealing treatment for copper foil? Optimize acm-BN Growth Success



















