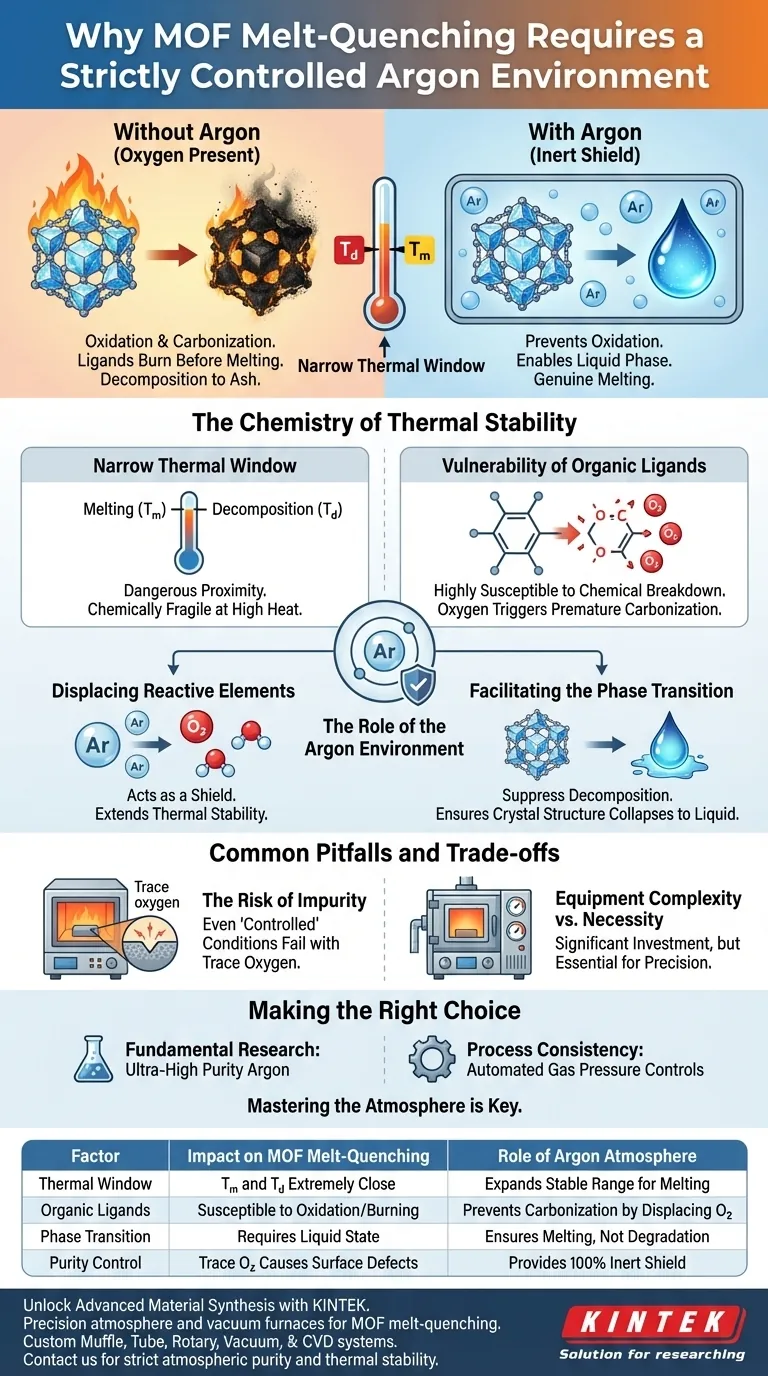
Strict atmospheric control is mandatory in MOF melt-quenching because the melting points of these materials are often dangerously close to their thermal decomposition temperatures. An atmosphere furnace using high-purity argon creates an oxygen-free environment that prevents the organic ligands within the framework from burning or carbonizing. Without this inert protection, the MOF would degrade chemically before it could physically transition into the liquid state required to form a glass.
The proximity of melting and decomposition temperatures in MOFs makes them chemically fragile at high heat. An inert argon atmosphere extends the thermal stability of organic ligands, allowing the material to melt into a liquid rather than degrade into ash.

The Chemistry of Thermal Stability
The Narrow Thermal Window
Many Metal-Organic Frameworks (MOFs), particularly the ZIF series, possess a unique thermal characteristic. Their melting point ($T_m$) and their decomposition temperature ($T_d$) are often separated by a very small margin.
Vulnerability of Organic Ligands
MOFs are hybrid materials containing organic linkers. These organic components are highly susceptible to chemical breakdown when exposed to heat.
If oxygen is present, these ligands will undergo oxidation immediately. This leads to premature carbonization, effectively destroying the crystal structure before melting can occur.
The Role of the Argon Environment
Displacing Reactive Elements
Using high-purity argon acts as a shield. It displaces oxygen and moisture that would otherwise react with the heated organic components.
This allows you to push the temperature higher without triggering immediate chemical decomposition.
Facilitating the Phase Transition
The ultimate goal of melt-quenching is to turn the crystalline MOF into a liquid (metal-organic liquid). This liquid state is the prerequisite for forming a glass upon cooling.
By suppressing decomposition, the argon atmosphere ensures the crystal structure has time to collapse into a disordered liquid state rather than burning away.
Common Pitfalls and Trade-offs
The Risk of Impurity
Even a furnace with "controlled" conditions can fail if gas purity is not absolute. Trace amounts of oxygen can catalyze decomposition at the surface of the sample, ruining the melt.
Equipment Complexity vs. Necessity
Atmosphere furnaces and vertical vacuum furnaces with gas pressure capabilities are significant investments. However, they are necessary to achieve the strictly controlled conditions required for this process.
While simpler heating methods exist, they lack the precision to navigate the narrow window between melting and destroying the MOF.
Making the Right Choice for Your Experiment
To achieve a successful glass transition, evaluate your specific requirements:
- If your primary focus is fundamental research: Prioritize ultra-high purity argon sources to ensure that any observed phase transition is genuine melting, not decomposition.
- If your primary focus is process consistency: Utilize a furnace with automated gas pressure controls to maintain a uniform inert environment across repeated cycles.
Mastering the atmosphere is the only way to unlock the liquid phase of these complex materials.
Summary Table:
| Factor | Impact on MOF Melt-Quenching | Role of Argon Atmosphere |
|---|---|---|
| Thermal Window | $T_m$ and $T_d$ are extremely close | Expands the stable thermal range for melting |
| Organic Ligands | Highly susceptible to oxidation and burning | Prevents carbonization by displacing oxygen |
| Phase Transition | Requires liquid state before cooling | Ensures material melts rather than degrading to ash |
| Purity Control | Trace oxygen causes surface decomposition | Provides a 100% inert shield for chemical integrity |
Unlock Advanced Material Synthesis with KINTEK
Precision is non-negotiable when navigating the narrow thermal windows of Metal-Organic Frameworks. KINTEK provides industry-leading atmosphere and vacuum furnace systems designed specifically to handle the delicate balance of MOF melt-quenching.
Backed by expert R&D and world-class manufacturing, we offer customizable Muffle, Tube, Rotary, Vacuum, and CVD systems tailored to your unique laboratory requirements. Don't let oxidation ruin your research—partner with KINTEK for equipment that guarantees strict atmospheric purity and thermal stability.
Ready to elevate your lab's capabilities? Contact us today to discuss your custom furnace needs!
Visual Guide
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- What is a furnace retort? Unlock Precise Atmospheric Control for Your Processes
- What is a retort furnace and what is its primary purpose? Master Controlled Heat Treatment for Your Materials
- What heat treatment processes require an inert atmosphere furnace? Ensure Pristine Finishes and Precise Control
- What are the key advantages of using atmosphere furnaces? Boost Efficiency and Control in Heat Treatment
- What industries commonly use inert atmosphere heat treating? Key Applications in Military, Automotive, and More
- What is an atmosphere box furnace and what are its primary uses? Essential for Controlled Heat Processing
- What safety features are typically included in controlled atmosphere furnaces? Ensure Safe Operation with Advanced Protection
- What are the advantages of using industrial-grade plasma nitriding furnaces? Boost Stainless Steel Surface Hardness



















