Graphite electrodes and tungsten wire igniters serve as the external activation mechanism that converts electrical energy into intense, localized thermal energy to start the reaction. This assembly heats only one end of the reactant compact until it reaches a specific ignition temperature. Once this threshold is crossed, a vigorous exothermic reaction between tungsten trioxide ($WO_3$) and magnesium ($Mg$) is triggered, releasing sufficient internal heat to sustain the process independently without further electrical input.
The ignition system acts solely as a catalyst to bridge the energy gap; once the local reaction begins, the material’s own chemical potential takes over to drive the carbonization wave through the entire compact.
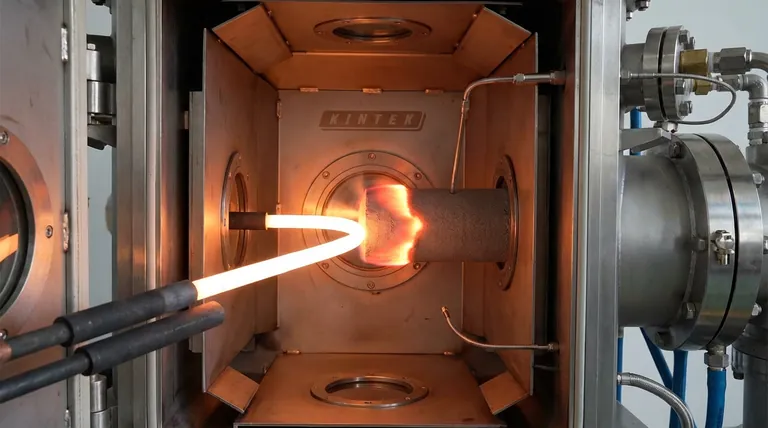
The Mechanism of Initiation
The initiation of Self-propagating High-temperature Synthesis (SHS) relies on a precise hand-off between external energy and internal chemical potential.
Electrical to Thermal Conversion
The process begins with graphite electrodes connected to a power source. These electrodes channel electrical current into tungsten wire igniters.
Because tungsten has high electrical resistance and a high melting point, the wire acts as a heating element. It rapidly converts the electrical energy into thermal energy.
Localized Heating
Unlike conventional sintering, which heats the entire furnace, this method applies heat locally.
The tungsten wire targets only one specific end of the reactant compact. This concentration of energy is efficient, ensuring that power is not wasted heating the entire volume of powder.
Reaching the Critical Threshold
The goal of the igniter is to raise the temperature of the reactants adjacent to the wire to the ignition point.
At this precise temperature, the kinetic barrier for the chemical reaction is broken. The external heating system effectively becomes obsolete the moment this chemical chain reaction begins.
The Propagation Phase
Once the ignition system has done its job, the physics of the process shift entirely to internal chemical dynamics.
The Exothermic Trigger
The primary driver of the synthesis is the reaction between tungsten trioxide ($WO_3$) and magnesium ($Mg$).
This specific chemical pairing is highly exothermic. When ignited, it releases a massive amount of heat energy almost instantaneously.
Sustaining the Wave
The heat generated by the initial $WO_3$ and $Mg$ reaction is not lost; it is transferred to the adjacent layer of unreacted powder.
This heat transfer triggers the reaction in the next layer, creating a self-propagating combustion wave. This wave travels through the compact, completing the carbonization process using the material's internal energy rather than external power.
Critical Operational Factors
While the ignition mechanism is straightforward, the environment in which it occurs is critical for safety and quality. Without controlling specific variables, the ignition can lead to failure rather than synthesis.
Managing Volatilization
The extreme heat generated during ignition and propagation can cause reactants to vaporize effectively destroying the stoichiometry of the product.
To prevent this, the process must occur within a high-pressure reactor. Introducing high-pressure argon gas (approximately 26 bar) creates a sealed environment that suppresses abnormal volatilization.
Structural Integrity
The reactor itself must be robust. The instantaneous pressure release from the exothermic reaction can spike up to 150 bar.
The containment vessel ensures that this pressure does not disrupt the stable propagation of the combustion wave.
Monitoring Extreme Temperatures
The reaction generates temperatures exceeding 2300°C, which is beyond the limit of standard sensors.
To accurately monitor the combustion front and analyze carbon loss kinetics, a Tungsten-Rhenium thermocouple (W/Re-20) is required. This specialized sensor captures real-time temperature distributions that standard thermal couples cannot survive.
Making the Right Choice for Your Goal
When designing or operating an SHS setup for tungsten carbide, understanding the relationship between the igniter and the environment is key.
- If your primary focus is Process Stability: Ensure your reactor maintains a consistent high-pressure argon atmosphere (approx. 26 bar) to prevent reactant loss during the volatile ignition phase.
- If your primary focus is Energy Efficiency: Rely on the igniter only for the initial trigger; optimize the reactant mix ($WO_3$ + $Mg$) to ensure the exothermic output is sufficient to sustain the wave without auxiliary heating.
The success of the process depends not just on the spark, but on containing the immense chemical energy that follows.
Summary Table:
| Component | Primary Role in SHS Process | Key Specification/Requirement |
|---|---|---|
| Graphite Electrodes | Current Conduction | Reliable electrical power transmission |
| Tungsten Wire | Localized Thermal Ignition | High melting point & electrical resistance |
| Reactant Mix | Internal Energy Source | $WO_3$ + $Mg$ (highly exothermic) |
| Argon Atmosphere | Pressure Management | ~26 bar to suppress volatilization |
| W/Re-20 Thermocouple | Thermal Monitoring | Capable of measuring >2300°C |
Elevate Your Material Synthesis with KINTEK
Precision in High-Temperature Synthesis requires more than just a spark—it demands a controlled environment and robust hardware. KINTEK provides industry-leading laboratory solutions tailored for advanced chemical processes.
Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, along with other specialized lab high-temp furnaces, all fully customizable to meet your unique SHS or carbonization needs.
Ready to optimize your synthesis workflow? Contact us today to discover how our high-pressure reactors and precision heating systems can enhance your lab's efficiency and product quality.
References
- Carbon Loss and Control for WC Synthesis through a Self-propagating High-Temperature WO3-Mg-C System. DOI: 10.1007/s11665-025-10979-z
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What advantage do silicon carbide heating elements have over traditional metal heating elements? Unlock High-Temp, Durable Performance
- What are the main advantages of graphite heating elements in vacuum ovens? Unlock Extreme Heat & Purity
- What are the common applications of DM Type Silicon Carbide Heating Elements? Versatile Solutions for High-Temp Processes
- How are heating elements designed for different appliances? Optimize Your Heating Solutions with Expert Design
- What is the maximum operating temperature of MoSi2 heating elements? Unlock High-Temp Performance & Longevity
- What roles do the Molybdenum container and Tantalum radiation shields play? Expert Knudsen Effusion Experiment Guide
- What are the common types of ceramic heating elements? Find the Right Heater for Your Application
- What is the composition and key properties of Nickel-Chromium (NiCr) alloys? Discover High-Performance Heating Solutions



















