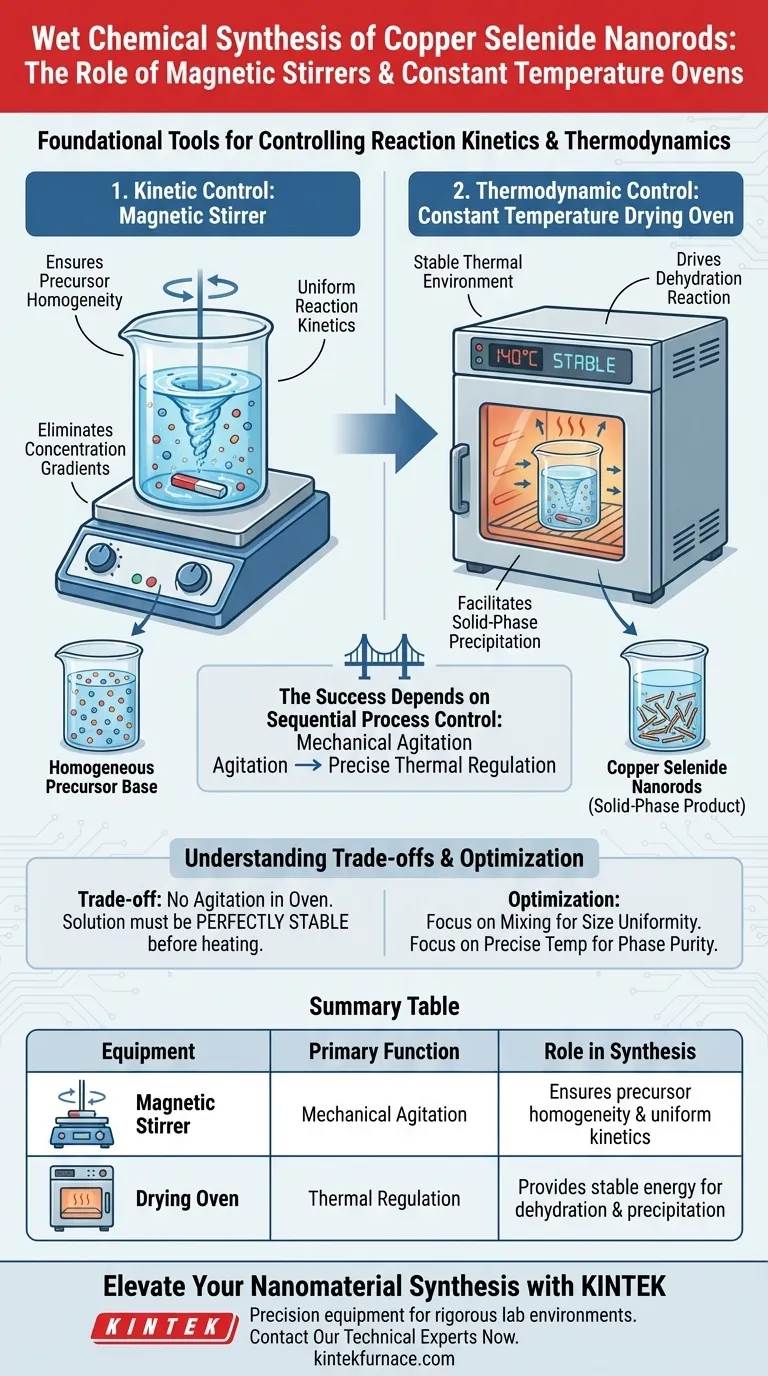
Magnetic stirrers and constant temperature ovens are the foundational tools for controlling reaction kinetics and thermodynamics in the wet chemical synthesis of copper selenide nanorods. The magnetic stirrer ensures the complete homogeneity of the precursor solution, while the constant temperature oven provides the stable thermal energy required to drive the specific dehydration reaction that precipitates solid-phase nanorods.
The success of this synthesis method depends on sequential process control: mechanical agitation first establishes a uniform chemical environment, followed by precise thermal regulation to force the phase transformation from liquid precursors to solid nanostructures.

The Role of Homogeneity in Precursor Preparation
Ensuring Uniform Reaction Kinetics
The magnetic stirrer is utilized during the initial phase to blend raw material solutions. Its primary function is to eliminate concentration gradients within the fluid.
By ensuring the solution is perfectly homogeneous, you guarantee that consistent reaction kinetics apply throughout the entire volume. Without this agitation, localized differences in concentration could lead to uneven growth rates or varying particle sizes.
Creating a Consistent Precursor Base
Before heat is applied, the reactants must be uniformly dispersed. The magnetic stirrer mechanically integrates the components, preparing the solution for the subsequent chemical transformation.
This step is critical because the quality of the final solid product is directly dependent on the uniformity of the liquid precursor mix.
Thermal Control and Phase Transformation
Providing a Steady Thermal Environment
Once the solution is mixed, it is transferred to an industrial-grade constant temperature drying oven. The oven is utilized to maintain a specific, non-fluctuating temperature, such as 140°C.
This steady thermal environment is essential. Fluctuations in temperature during this stage can alter the crystallization process, leading to defects in the nanorods or incomplete reactions.
Driving the Dehydration Reaction
The heat provided by the oven serves as the catalyst for a specific chemical change: the dehydration reaction.
This process removes water from the precursor structure. It provides the energy barrier needed for the precursors to chemically transform from a dissolved state into a solid state.
Facilitating Solid-Phase Precipitation
As the dehydration reaction progresses under constant heat, the precursors precipitate.
This precipitation results in the formation of solid-phase products, specifically the copper selenide nanorods. The stability of the oven ensures that this precipitation occurs at a controlled rate, which is vital for achieving the desired nanorod morphology.
Understanding the Trade-offs
The Separation of Mixing and Heating
A key limitation of using a drying oven is the lack of agitation during the heating phase. Once the vessel is inside the oven, the magnetic stirrer is typically no longer in play.
This means the solution must be perfectly stable before entering the oven. If the precursors settle or segregate before the temperature reaches the reaction point (e.g., 140°C), the resulting nanorods may be inconsistent.
Kinetic vs. Thermodynamic Dependencies
The magnetic stirrer addresses kinetic issues (how fast and how well things mix), while the oven addresses thermodynamic issues (providing energy for phase change).
You cannot compensate for poor mixing with better heating. If the magnetic stirring phase is rushed or inadequate, the constant temperature of the oven will simply lock those imperfections into the final solid product.
Optimizing the Synthesis Process
To ensure high-quality copper selenide nanorods, apply these principles based on your specific process goals:
- If your primary focus is size uniformity: Prioritize the magnetic stirring phase to ensure the raw materials are completely homogenized before any heat is applied.
- If your primary focus is phase purity: Ensure the drying oven is calibrated to hold the target temperature (e.g., 140°C) precisely, as this drives the completeness of the dehydration reaction.
precise coordination of mechanical mixing and thermal stability is what transforms raw chemicals into structured nanomaterials.
Summary Table:
| Equipment Type | Primary Function | Role in Synthesis |
|---|---|---|
| Magnetic Stirrer | Mechanical Agitation | Ensures precursor homogeneity and uniform reaction kinetics. |
| Drying Oven | Thermal Regulation | Provides stable energy (e.g., 140°C) for the dehydration reaction. |
| Process Step | Mechanism | Output |
| Pre-heating Phase | Concentration Gradient Removal | Perfectly blended, stable liquid precursor base. |
| Heating Phase | Constant Thermal Environment | Controlled solid-phase precipitation of nanorods. |
Elevate Your Nanomaterial Synthesis with KINTEK
Precision is the difference between inconsistent results and high-performance nanostructures. Backed by expert R&D and manufacturing, KINTEK offers high-performance magnetic stirrers and advanced constant temperature ovens designed for rigorous lab environments.
Whether you require Muffle, Tube, Rotary, Vacuum, or CVD systems, our laboratory high-temp furnaces are fully customizable to meet your unique synthesis needs. Ensure perfect phase purity and morphology for your copper selenide nanorods today.
Contact Our Technical Experts Now
Visual Guide
References
- Rajesh Rajasekharan, Manikoth M. Shaijumon. Bifunctional Current Collectors for Lean‐Lithium Metal Batteries. DOI: 10.1002/adfm.202502473
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- How does Faraday's Law of Induction work in induction heating? Achieve Precise, Non-Contact Thermal Processing
- Why is a 105 °C drying process in an electric drying oven significant? Prevent Refractory Structural Failure
- What is the significance of applying full displacement constraints at fixed entry points? Ensure Thermal Accuracy
- What is the purpose of the constant-temperature circulation phase? Ensure Moso Bamboo Integrity with KINTEK Solutions
- What are batch catalytic debinding ovens used for? Speed Up MIM/CIM with Low-Temp Debinding
- Which type of furnace is better for specific applications? Choose the Right Furnace for Your Production Needs
- How is SEM utilized to evaluate manganese phosphate catalysts after calcination? Verify Nanosheet Integrity.
- What is the function of a Mass Flow Controller (MFC)? Achieve Precise Ethanol Vapor Delivery for Graphene Synthesis



















