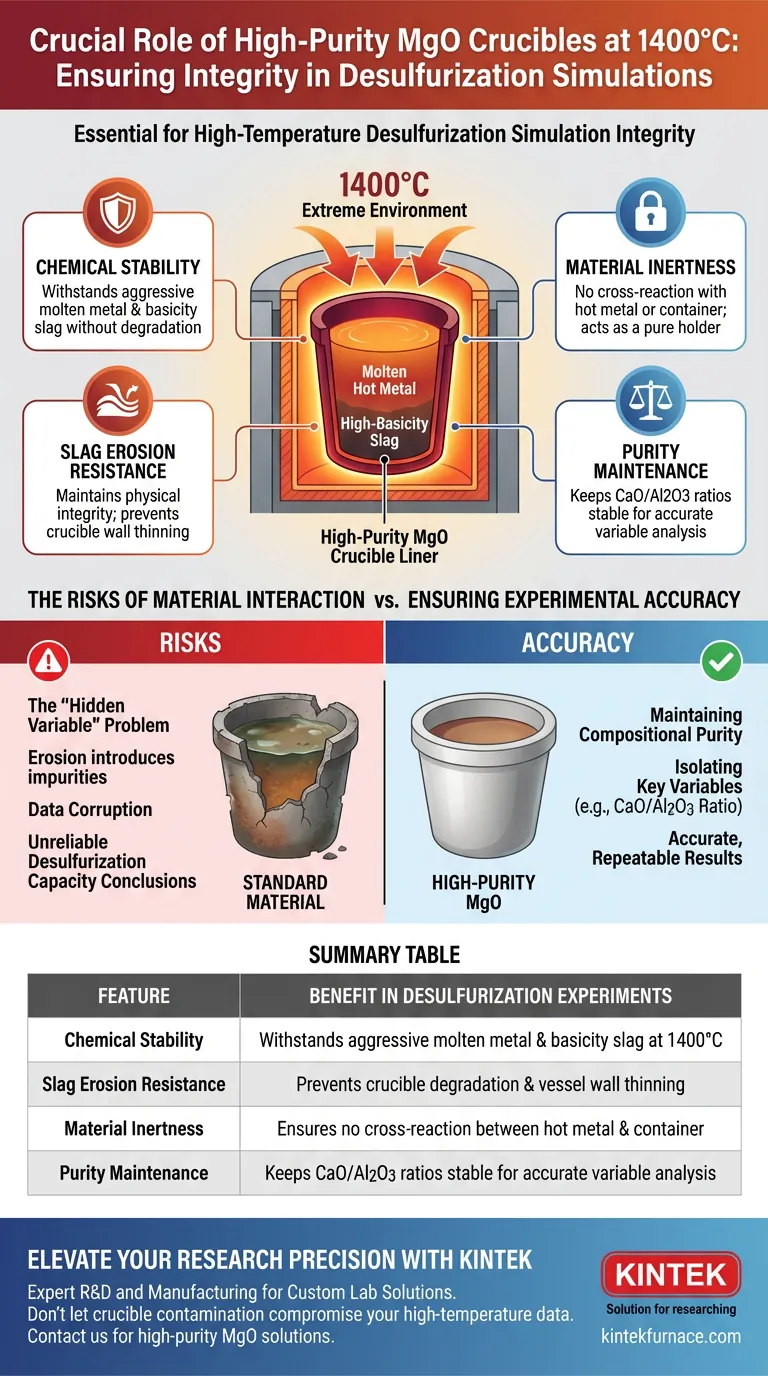
High-purity magnesium oxide (MgO) crucibles are strictly required to ensure the integrity of data in high-temperature desulfurization simulations at 1400°C. Their primary function is to provide exceptional chemical stability and resistance to slag erosion, preventing the vessel itself from reacting with the molten hot metal or high-basicity slag. This isolation is critical for preventing contamination of the slag's chemical composition during the experiment.
The choice of crucible is not merely about structural containment; it is about chemical isolation. Using an MgO liner ensures that the slag composition remains unaltered, allowing you to attribute changes in desulfurization capacity solely to your experimental variables, such as the CaO/Al2O3 ratio.
The Critical Role of Chemical Stability
Withstanding Extreme Environments
At 1400°C, standard materials often fail due to the aggressive nature of molten metals. High-purity MgO crucibles possess excellent chemical stability capable of enduring these thermal conditions without degrading.
Resisting Slag Erosion
High-basicity desulfurization slags are highly corrosive to many refractory materials. MgO liners are specifically selected for their ability to resist slag erosion, maintaining their physical integrity throughout the simulation.
Preventing Cross-Reactions
The validity of the experiment depends on the container remaining inert. MgO prevents chemical reactions between the molten hot metal and the crucible wall. This ensures the vessel acts only as a holder, not a participant in the chemical process.
Ensuring Experimental Accuracy
Maintaining Compositional Purity
For a simulation to be valid, the chemistry of the slag must essentially remain constant relative to external inputs. An MgO crucible ensures the chemical composition of the desulfurization slag is not contaminated by dissolving refractory material.
Isolating Key Variables
Researchers often need to evaluate how specific parameters, such as the CaO/Al2O3 ratio, influence performance. If the crucible reacts with the slag, it alters this ratio unpredictably. MgO allows for an accurate evaluation of desulfurization capacity by keeping the baseline environment stable.
The Risks of Material Interaction
The "Hidden Variable" Problem
A common pitfall in metallurgical simulations is failing to account for the container as a source of error. If a less stable material were used, the crucible would erode and introduce impurities into the melt.
Data Corruption
When the container reacts with the slag, the resulting data reflects a mixture of the intended experiment and the dissolving crucible. This renders any conclusions regarding the desulfurization capacity suspect or entirely invalid.
Making the Right Choice for Your Goal
To ensure your high-temperature simulations yield valid, publishable results, align your material selection with your specific analytical needs:
- If your primary focus is Chemical Purity: Use high-purity MgO to prevent the container from chemically altering the molten hot metal or slag.
- If your primary focus is Variable Analysis: Rely on MgO to ensure that observed changes in performance are due to the CaO/Al2O3 ratio, not crucible erosion.
Select MgO liners to transform your crucible from a potential variable into a reliable constant.
Summary Table:
| Feature | Benefit in Desulfurization Experiments |
|---|---|
| Chemical Stability | Withstands aggressive molten metal and basicity slag at 1400°C |
| Slag Erosion Resistance | Prevents crucible degradation and vessel wall thinning |
| Material Inertness | Ensures no cross-reaction between hot metal and container |
| Purity Maintenance | Keeps CaO/Al2O3 ratios stable for accurate variable analysis |
Elevate Your Research Precision with KINTEK
Don't let crucible contamination compromise your high-temperature data. KINTEK provides high-purity MgO solutions designed to withstand the most corrosive metallurgical environments.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, CVD systems, and other lab high-temp furnaces, all customizable for your unique experimental needs. Whether you are analyzing desulfurization capacity or testing new slag compositions, our equipment ensures your results are accurate and repeatable.
Ready to optimize your thermal processes? Contact us today to find your custom lab solution.
Visual Guide
References
- Jyun-Ming Shen, Weite Wu. Effects of Different CaO/Al2O3 Ratios on the Phase Composition and Desulfurization Ability of CaO-Based Desulfurizers in Hot Metal. DOI: 10.3390/met14030363
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Magnesium Extraction and Purification Condensing Tube Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1200℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What is the purpose of a water-cooling jacket in a methane cracking reactor? Prevent Blockages & Thermal Damage
- How do high-precision mass flow controllers assist in the formation of superlattice structures? Mastery of 2D CVD
- What is the function of molybdenum fixtures in high-temperature heat treatment? Ensure Perfect Diffusion Integrity
- What is the primary function of an alumina crucible in preparing B2O3–ZnO–BaO shielding glass? Process Secrets Revealed
- Why must a rotary vane vacuum pump be integrated into the curing platform for phenolic laminates? Key to Void-Free Parts
- What creates the pumping action in a circulating water vacuum pump? Discover the Liquid Ring Mechanism
- Why are high-purity alumina crucibles preferred over quartz crucibles at 1873 K? Ensure Precision at Extreme Heat
- Why is the pore size of refractory materials significant? Unlocking Precision in Bubble Formation and Oxygen Impact



















