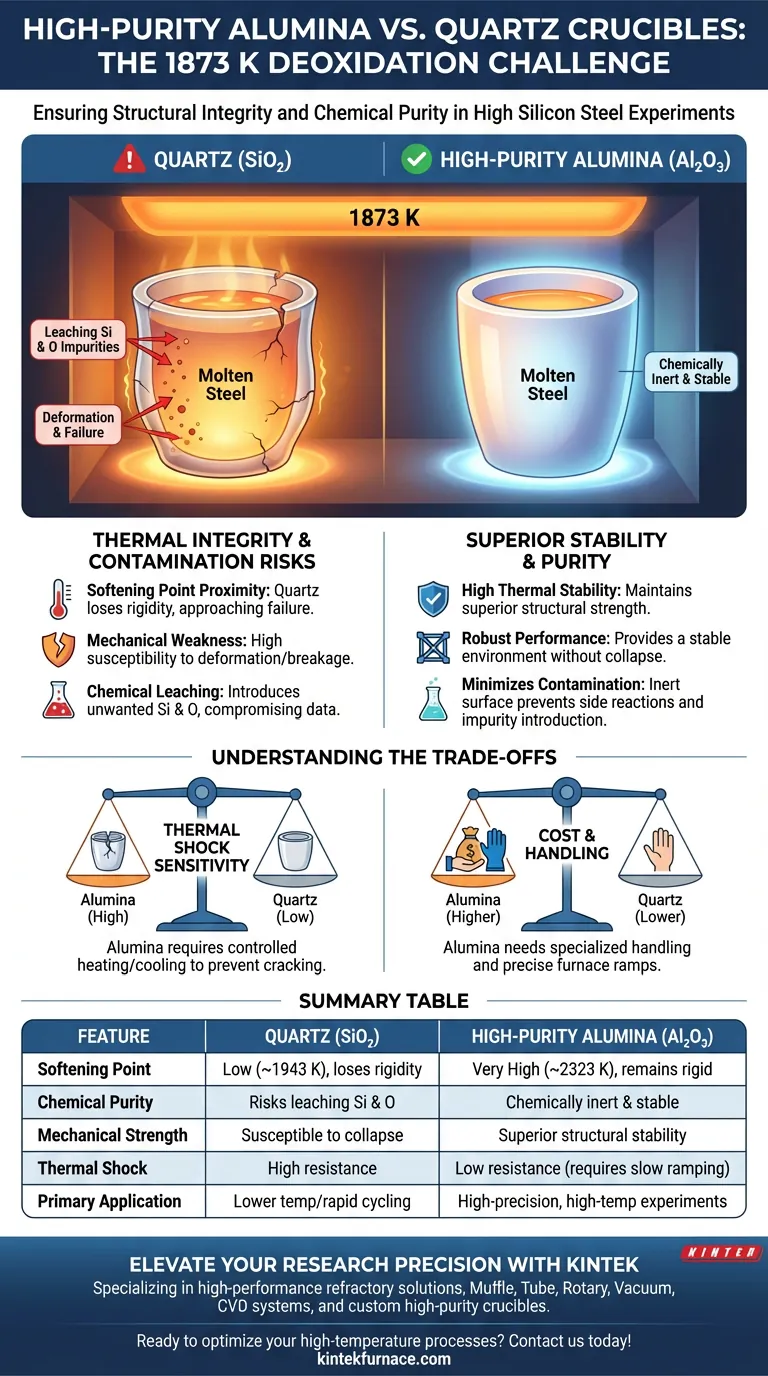
At the extreme temperature of 1873 K, the choice of crucible material dictates the physical integrity and chemical purity of the steel melt. High-purity alumina is preferred because it maintains structural rigidity and chemical inertness, whereas quartz approaches its softening point, leading to mechanical failure and significant melt contamination.
Selecting alumina crucibles ensures experimental precision by preventing the structural collapse and chemical leaching that occur when quartz is pushed to its thermal limits at 1873 K.

Thermal Integrity at Extreme Temperatures
The Softening Point of Quartz
At 1873 K, quartz (silicon dioxide) operates dangerously close to its softening point. This proximity causes the material to lose its structural rigidity, making it highly susceptible to deformation or breakage during the experiment.
The Superior Strength of Alumina
In contrast, high-purity alumina exhibits superior thermal stability and mechanical strength at these elevated temperatures. It remains physically robust, providing a stable environment for high silicon steel deoxidation without the risk of vessel collapse.
Minimizing Chemical Contamination
Preventing Unwanted Impurity Introduction
When quartz deforms at high temperatures, it can introduce unwanted silicon and oxygen impurities into the steel melt. This contamination compromises the experimental data, as it becomes impossible to distinguish between added silicon and silicon leached from the crucible.
Reducing Side Reactions via Surface Stability
High-purity alumina maintains a smooth and intact surface throughout the heating process. This physical consistency significantly reduces the likelihood of side reactions between the crucible wall and the molten steel.
Understanding the Trade-offs
Thermal Shock Sensitivity
While alumina is structurally superior at 1873 K, it is more sensitive to thermal shock than quartz. This means heating and cooling cycles must be carefully controlled to prevent the alumina from cracking due to rapid temperature changes.
Material Compatibility and Cost
High-purity alumina is generally more expensive than quartz and may require specialized handling. Researchers must balance the need for high-purity results with the higher operational costs and the necessity of precise furnace ramp rates.
How to Apply This to Your Project
Before beginning a high-temperature deoxidation experiment, evaluate your material choice based on the following priorities:
- If your primary focus is Chemical Precision: Utilize high-purity alumina to ensure that no exogenous silicon or oxygen alters your melt composition.
- If your primary focus is Structural Reliability: Choose alumina for its high mechanical strength at 1873 K to prevent crucible deformation during long-duration experiments.
- If your primary focus is Rapid Thermal Cycling: Exercise caution with alumina and implement slow heating rates to mitigate its inherent sensitivity to thermal shock.
Choosing the right refractory material is the first step in ensuring that your experimental results reflect the chemistry of the steel rather than the limitations of the container.
Summary Table:
| Feature | Quartz (SiO2) | High-Purity Alumina (Al2O3) |
|---|---|---|
| Softening Point | Low (~1943 K), loses rigidity at 1873 K | Very High (~2323 K), remains rigid |
| Chemical Purity | Risks leaching Si and O into the melt | Chemically inert and stable |
| Mechanical Strength | Susceptible to deformation/collapse | Superior structural stability |
| Thermal Shock | High resistance | Low resistance (requires slow ramping) |
| Primary Application | Lower temp or rapid cycling | High-precision, high-temp experiments |
Elevate Your Research Precision with KINTEK
Don't let crucible failure compromise your experimental data. At KINTEK, we specialize in high-performance refractory solutions designed for the most demanding thermal environments. Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with customizable high-temperature lab furnaces and high-purity crucibles tailored to your unique specifications. Whether you are conducting high silicon steel deoxidation or advanced material synthesis, our technical experts are ready to help you select the ideal materials and equipment for success.
Ready to optimize your high-temperature processes? Contact us today to discuss your custom lab needs!
Visual Guide
References
- Sanjay Pindar, Manish M. Pande. Influence of Ferrosilicon Addition on Silicon-oxygen Equilibria in High-silicon Steels. DOI: 10.2355/isijinternational.isijint-2024-018
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What processes is the circulating water multifunctional vacuum pump suitable for? Ideal for Clean, Economical Lab Vacuum Needs
- What is a vacuum chamber good for? Mastering Material Processing with Environmental Control
- What are the main components of a Laboratory Furnace? Essential Parts for Precise High-Temperature Processing
- Why is a BN coating used in Mg3Sb2 melting? Essential Purity and Protection Guide
- Why is a high-pressure MFC necessary for CHP systems? Achieve Precision in Catalytic Hydropyrolysis Data
- What are the advantages of using a single-mode microwave generator? Precision Heating for Metal Recovery
- What is the function of high-purity alumina crucibles? Protect Samples and Furnaces During Oxide Calcination
- How does the integration of digital control panels and safety devices enhance industrial electric furnace operation?



















