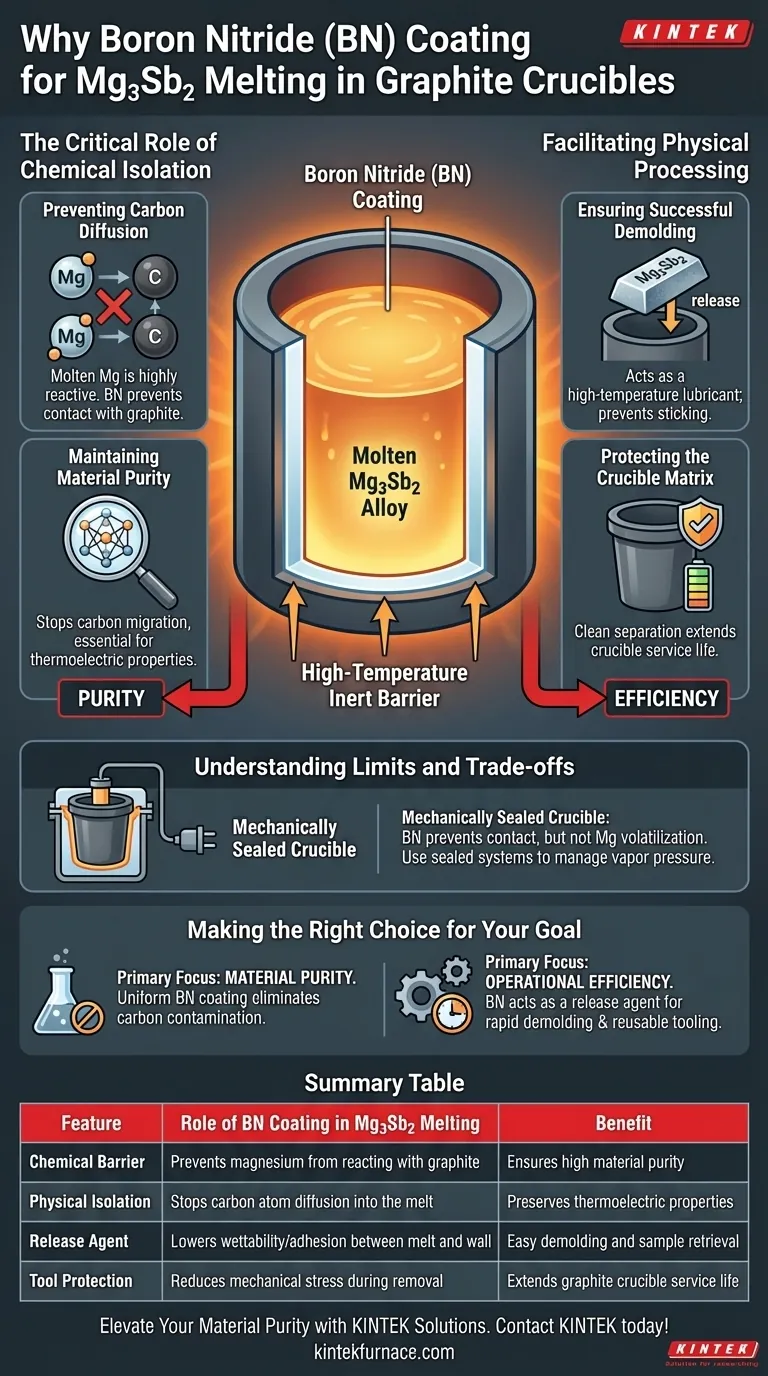
The primary function of a Boron Nitride (BN) coating is to serve as a high-temperature inert barrier. When melting Magnesium-Antimony (Mg3Sb2), the coating is applied to the inner walls of the graphite crucible to physically and chemically isolate the molten alloy from the carbon matrix. This prevents the reactive magnesium from bonding with the graphite, ensuring the final material remains pure and free of carbon contamination.
Core Takeaway By acting as a non-reactive shield, Boron Nitride solves the dual problem of chemical contamination and mechanical adhesion. It preserves the purity of the thermoelectric material by stopping carbon diffusion and functions as a release agent to ensure the solidified sample can be removed without damaging the crucible.

The Critical Role of Chemical Isolation
Preventing Carbon Diffusion
The most significant risk during the melting process is the introduction of impurities. Molten magnesium is highly reactive and will readily interact with a bare graphite surface.
Maintaining Material Purity
The BN coating creates a robust interface that prevents carbon atoms from migrating into the Mg3Sb2 melt. This is essential for thermoelectric applications, where even trace carbon impurities can severely degrade the material's performance and electronic properties.
Facilitating Physical Processing
Ensuring Successful Demolding
Beyond chemical protection, the BN coating acts as a high-temperature lubricant or release agent. Molten alloys often exhibit high wettability, meaning they tend to stick or fuse to graphite surfaces upon cooling.
Protecting the Crucible Matrix
Without this isolation layer, removing the solidified sample would likely require mechanical force that could damage the graphite tool. The coating ensures the sample separates cleanly, extending the service life of the graphite crucible and allowing for repeated usage.
Understanding the Limits and Trade-offs
The Scope of Protection
While BN effectively stops chemical reactions, it does not solve every processing challenge. For example, Boron Nitride prevents contact interactions, but it does not inherently prevent the volatilization of magnesium vapor.
Managing Magnesium Volatility
Magnesium has a high vapor pressure and evaporates easily at melting temperatures. To address this, the BN coating is often used in conjunction with a mechanically sealed crucible system (such as one equipped with a plug). The BN handles the purity and adhesion, while the physical seal maintains the stoichiometric stability of the alloy.
Making the Right Choice for Your Goal
- If your primary focus is Material Purity: Ensure the BN coating is applied uniformly to prevent any direct contact between the melt and the graphite, which eliminates the risk of carbon contamination.
- If your primary focus is Operational Efficiency: Utilize the BN coating as a release agent to facilitate rapid demolding and maximize the reusable lifespan of your graphite tooling.
Summary: The application of Boron Nitride is a non-negotiable step in Mg3Sb2 processing that safeguards the chemical integrity of the alloy while preserving the physical integrity of the casting equipment.
Summary Table:
| Feature | Role of BN Coating in Mg3Sb2 Melting | Benefit |
|---|---|---|
| Chemical Barrier | Prevents magnesium from reacting with graphite | Ensures high material purity |
| Physical Isolation | Stops carbon atom diffusion into the melt | Preserves thermoelectric properties |
| Release Agent | Lowers wettability/adhesion between melt and wall | Easy demolding and sample retrieval |
| Tool Protection | Reduces mechanical stress during removal | Extends graphite crucible service life |
Elevate Your Material Purity with KINTEK Solutions
Precise thermal processing is the backbone of advanced material science. At KINTEK, we understand that every detail—from inert coatings to stoichiometric stability—matters. Backed by expert R&D and world-class manufacturing, we provide high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, along with customizable lab high-temperature furnaces designed to meet your most rigorous research needs.
Whether you are refining thermoelectric alloys like Mg3Sb2 or developing next-generation ceramics, our technical team is ready to help you optimize your process.
Contact KINTEK today to discuss your custom furnace requirements!
Visual Guide
Related Products
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What role does a high-precision constant temperature drying oven play in battery electrode preparation? Master Battery Performance
- What is the range of internal volumes for Laboratory Type Furnaces? Choose the Right Size for Your Lab Needs
- What function do the piping and butterfly valve components serve in a multi-kiln carbonization system? Maximize Control
- What are the advantages of using high-purity alumina crucibles? Achieve Precise Cast Iron Phase Equilibrium Data
- What is the purpose of waveguide-to-coax adapters? Key Roles in High-Temperature Measurement Chains
- How is quartz wool utilized in the assembly of reaction tubes? Optimize Crystal Growth and Flux Separation
- What is aluminosilicate wool (ASW) and its typical application temperature range? Discover High-Temp Insulation Solutions
- What is the function of a vacuum ampoule during the synthesis of ZnGeP2? Ensure Purity and Chemical Stability



















