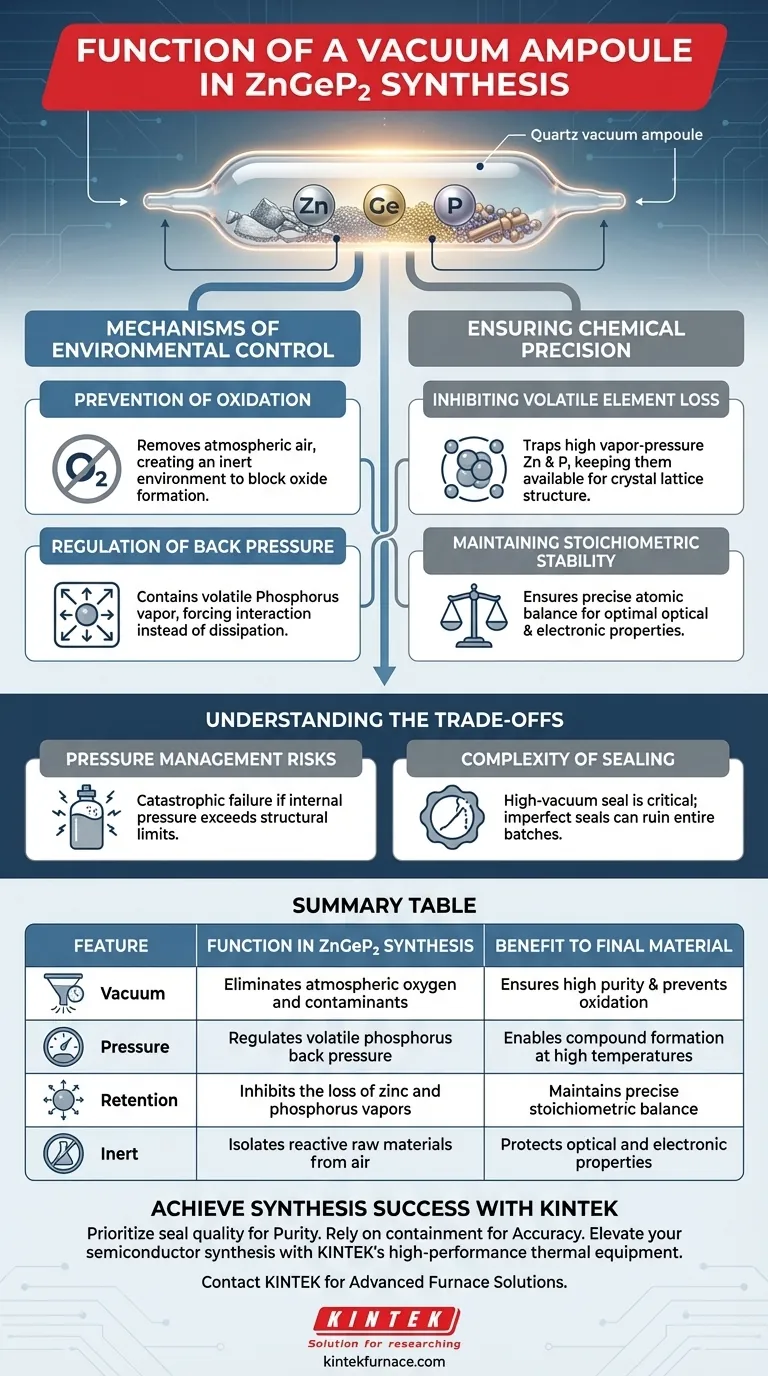
The primary function of a vacuum ampoule during Zinc Germanium Phosphide (ZnGeP2) synthesis is to serve as a hermetically sealed containment vessel that preserves chemical integrity. By encapsulating raw zinc, germanium, and phosphorus under vacuum, the ampoule isolates the reaction from the outside environment and creates the necessary internal pressure conditions for successful compound formation.
Synthesis of ZnGeP2 involves heating volatile elements that would otherwise escape or degrade. The vacuum ampoule solves this by preventing oxidation and trapping vapors, ensuring the final material maintains the precise chemical balance necessary for semiconductor applications.

Mechanisms of Environmental Control
Prevention of Oxidation
At the high temperatures required for synthesis, raw materials are highly reactive. Oxygen exposure during this phase would lead to immediate degradation.
The vacuum ampoule removes atmospheric air prior to sealing. This creates an inert environment that effectively blocks the formation of unwanted oxides, ensuring the purity of the final compound.
Regulation of Back Pressure
Phosphorus, a key component of ZnGeP2, is highly volatile. When heated, it creates significant internal vapor pressure.
The ampoule is designed to withstand and regulate this "back pressure." By containing the expanding gas, the ampoule forces the phosphorus to interact with the zinc and germanium rather than dissipating.
Ensuring Chemical Precision
Inhibiting Volatile Element Loss
Without a sealed environment, elements with high vapor pressures—specifically zinc and phosphorus—would evaporate out of the reaction zone.
The vacuum ampoule physically traps these elements. This inhibition of loss ensures that the atoms remain available to form the crystal lattice structure.
Maintaining Stoichiometric Stability
"Stoichiometry" refers to the specific, ideal ratio of elements within a compound. High-performance ZnGeP2 requires an exact atomic balance.
By preventing the escape of volatile components, the ampoule ensures the final product retains this balance. This stability is critical for the material's optical and electronic properties.
Understanding the Trade-offs
Pressure Management Risks
While the ampoule is essential for containing phosphorus back pressure, this creates a mechanical challenge.
If the internal pressure exceeds the ampoule's structural limits, catastrophic failure can occur. The synthesis process relies heavily on the physical integrity of the ampoule walls.
Complexity of Sealing
The vacuum must be absolute to be effective. Imperfect seals do not just reduce efficiency; they can ruin the entire batch.
Achieving a high-vacuum seal requires precise preparation, adding a layer of complexity to the manufacturing workflow compared to open-system synthesis methods.
Achieving Synthesis Success
To maximize the quality of your ZnGeP2 synthesis, consider the following regarding ampoule usage:
- If your primary focus is Material Purity: Prioritize the quality of the vacuum seal to ensure zero oxygen ingress during the heating phase.
- If your primary focus is Stoichiometric Accuracy: Rely on the ampoule's ability to contain pressure to prevent the depletion of volatile phosphorus and zinc.
The vacuum ampoule is not merely a container; it is an active tool for thermodynamic control that defines the success of the synthesis process.
Summary Table:
| Feature | Function in ZnGeP2 Synthesis | Benefit to Final Material |
|---|---|---|
| Vacuum Sealing | Eliminates atmospheric oxygen and contaminants | Ensures high purity and prevents oxidation |
| Pressure Containment | Regulates volatile phosphorus back pressure | Enables compound formation at high temperatures |
| Element Retention | Inhibits the loss of zinc and phosphorus vapors | Maintains precise stoichiometric balance |
| Inert Environment | Isolates reactive raw materials from air | Protects optical and electronic properties |
Elevate Your Semiconductor Synthesis with KINTEK
Precise control over volatile elements like Phosphorus requires high-performance thermal equipment you can trust. KINTEK provides industry-leading Muffle, Tube, Rotary, Vacuum, and CVD systems, all designed to support the rigorous demands of material synthesis and crystal growth. Backed by expert R&D and manufacturing, our laboratory high-temperature furnaces are fully customizable to meet your unique chemical integrity and stoichiometric stability requirements.
Ready to optimize your ZnGeP2 synthesis? Contact KINTEK today to discover how our advanced furnace solutions can enhance your research and production outcomes.
Visual Guide
References
- Alexey Lysenko, Alexey Olshukov. Band-like Inhomogeneity in Bulk ZnGeP2 Crystals, and Composition and Influence on Optical Properties. DOI: 10.3390/cryst15040382
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Ultra-High Vacuum Flange Aviation Plug Glass Sintered Airtight Circular Connector for KF ISO CF
- Stainless Steel Quick Release Vacuum Chain Three Section Clamp
- Ultra High Vacuum Observation Window Stainless Steel Flange Sapphire Glass Sight Glass for KF
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- 304 316 Stainless Steel High Vacuum Ball Stop Valve for Vacuum Systems
People Also Ask
- What are the typical applications of a circulating water vacuum pump? Essential for Lab Efficiency and Cost Savings
- What is the primary function of a constant-temperature heating plate in TMD film transfer? Optimize Your 2D Material Process
- Why is toluene used as a grinding aid in wet ball milling? Master Fine Metal Powder Synthesis with PCAs
- Why use a fusion furnace and platinum crucibles for XRF analysis of magnesium slag? Ensure Accurate Results
- How does a laboratory drying oven ensure the structural stability of microcapsule granules? Expert Drying Guide
- Why is a glovebox environment necessary for KBaBi synthesis? Protect Sensitive Raw Materials Today
- Why use graphite or quartz crucibles for liquid antimony-tellurium? Protect Your High-Temp Melt Integrity
- How does a laboratory blast drying oven facilitate BCZT gel drying? Precision Solutions for High-Quality Xerogels

















