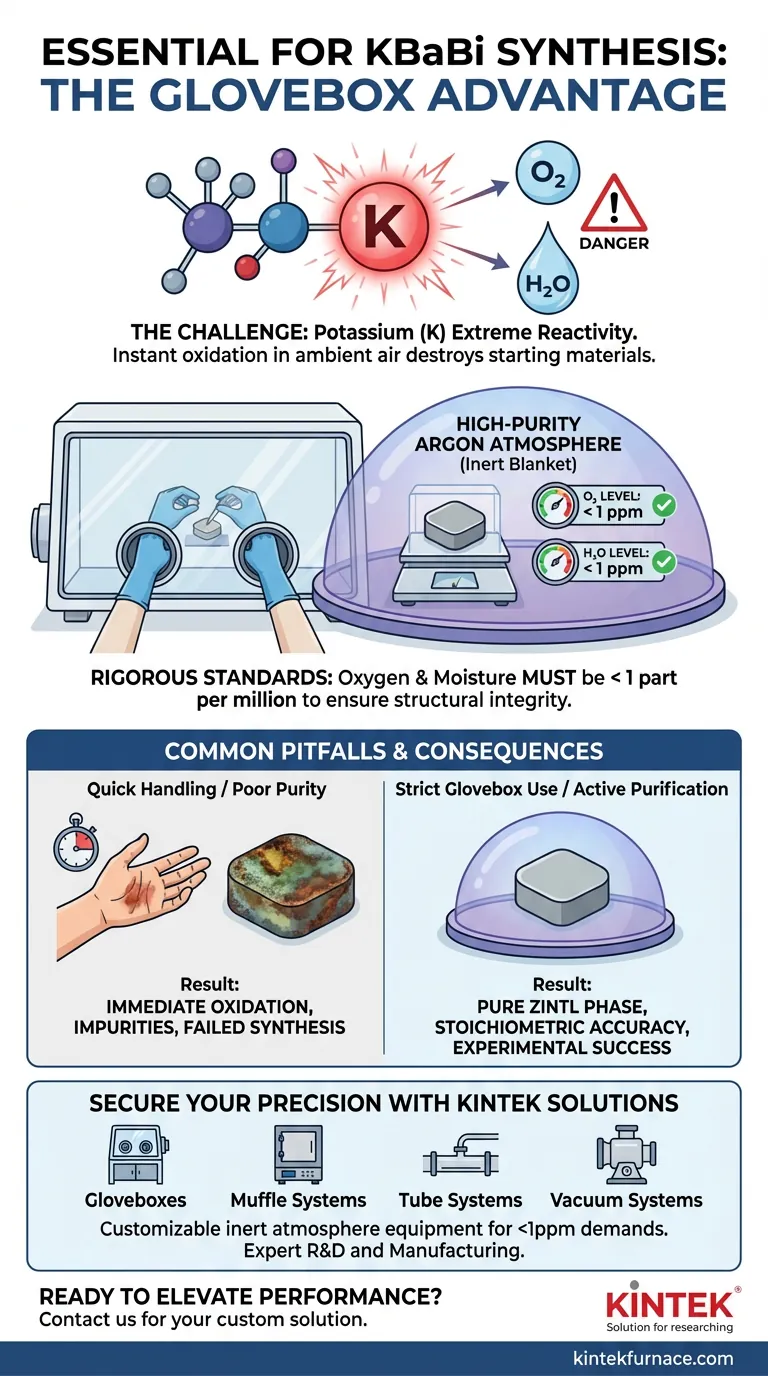
A glovebox environment is strictly mandatory for weighing raw materials in KBaBi synthesis due to the extreme chemical instability of potassium (K). This alkali metal reacts almost instantaneously upon contact with ambient air or humidity, making standard open-air handling impossible without destroying the starting material.
To prevent rapid oxidation and ensure the structural integrity of the final Zintl phase, raw materials must be handled in a high-purity argon atmosphere. Water and oxygen levels within this environment must be rigorously maintained below 1 part per million (ppm).
The Chemical Vulnerability of Potassium
Extreme Reactivity
Potassium is the primary driver for the strict environmental requirements in this synthesis. It is highly chemically active, possessing a strong tendency to donate an electron. This makes it aggressively reactive toward oxidizers found in normal air.
The Consequence of Exposure
If potassium is exposed to the atmosphere even briefly, it oxidizes. This reaction alters the stoichiometry of your raw materials before the synthesis even begins. Instead of a pure KBaBi Zintl phase, you will likely produce unwanted oxides or hydroxides, compromising the purity and electronic properties of the sample.
Creating the Necessary Conditions
High-Purity Argon Atmosphere
To neutralize the threat of oxidation, the glovebox must be filled with high-purity argon. Argon is a noble gas and remains inert even when in contact with highly reactive metals like potassium. It acts as a protective blanket during the cutting and weighing stages.
Rigorous Purity Standards
Simply filling a box with argon is insufficient; the quality of the atmosphere is critical. You must ensure that both water vapor and oxygen levels are maintained below 1 ppm. This ultra-low threshold is necessary because even trace amounts of moisture or oxygen can degrade the potassium surface over time.
Common Pitfalls to Avoid
The "Quick Handling" Fallacy
A common mistake is assuming that rapid weighing in air or a low-quality inert setup is acceptable. Potassium's reaction rate is fast enough that surface oxidation occurs almost immediately, introducing impurities that are difficult to remove later.
Relying on Insufficient Purity
Standard industrial-grade inert gas often contains moisture levels higher than 1 ppm. Without an active purification system (getters) to scrub the atmosphere, the environment will not be sufficiently inert to protect the potassium for the duration of the weighing process.
Ensuring Experimental Success
To guarantee the synthesis of high-quality KBaBi compounds, adhere to the following guidelines:
- If your primary focus is phase purity: Verify that your glovebox sensors confirm oxygen and moisture levels are strictly below 1 ppm before opening raw material containers.
- If your primary focus is process consistency: Utilize high-purity argon exclusively, avoiding nitrogen or other gases that might interact with the reagents at higher temperatures or specific conditions.
Strict control of the atmospheric environment is not merely a precaution; it is the fundamental baseline required to successfully synthesize these sensitive materials.
Summary Table:
| Environmental Factor | Requirement | Reason for Stringency |
|---|---|---|
| Primary Gas | High-Purity Argon | Provides an inert blanket; non-reactive with Potassium. |
| Oxygen Level | < 1 ppm | Prevents immediate surface oxidation and stoichiometric shifts. |
| Moisture Level | < 1 ppm | Avoids violent reactions with K and formation of hydroxides. |
| Handling Protocol | Strict Glovebox Use | Ensures raw material integrity from cutting to weighing. |
Secure Your Synthesis Precision with KINTEK
Don’t let atmospheric contamination compromise your Zintl phase research. KINTEK provides industry-leading inert atmosphere solutions designed for the most reactive materials. Backed by expert R&D and manufacturing, we offer high-performance Gloveboxes, Muffle, Tube, and Vacuum systems that are fully customizable to maintain the <1 ppm purity levels your KBaBi synthesis demands.
Ready to elevate your lab's performance? Contact us today to find your custom solution!
Visual Guide
References
- Investigation of a Ternary Zintl Phase KBaBi: Synthesis, Crystal Structure, and Preliminary Transport Properties. DOI: 10.1002/zaac.202500064
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What cost factors should be considered when choosing an alumina ceramic furnace tube? Optimize Total Cost of Ownership
- What is the primary function of a drying oven during LLZTO preparation? Ensure Pure Phase Solid Electrolytes
- What vacuum range can a circulating water vacuum pump achieve? Optimize Your Lab's Vacuum Performance
- Why are high-purity graphite molds essential for the sintering of Tin Selenide (SnSe) alloys? Key to Precise SPS Results
- Why are sealed Niobium (Nb) tubes utilized as reaction vessels during the high-temperature solid-state synthesis of Ba1-xEuxZn2Sb2?
- What is the purpose of a water circulating vacuum pump? Achieve Clean, Efficient Vacuum for Lab Processes
- Why is temperature-controlled heating equipment required for calcium perrhenate? Ensure Rhenium Stability at 140 °C
- What is the primary function of a high-purity vacuum-sealed quartz tube in the Modified Bridgman technique? Key Role



















