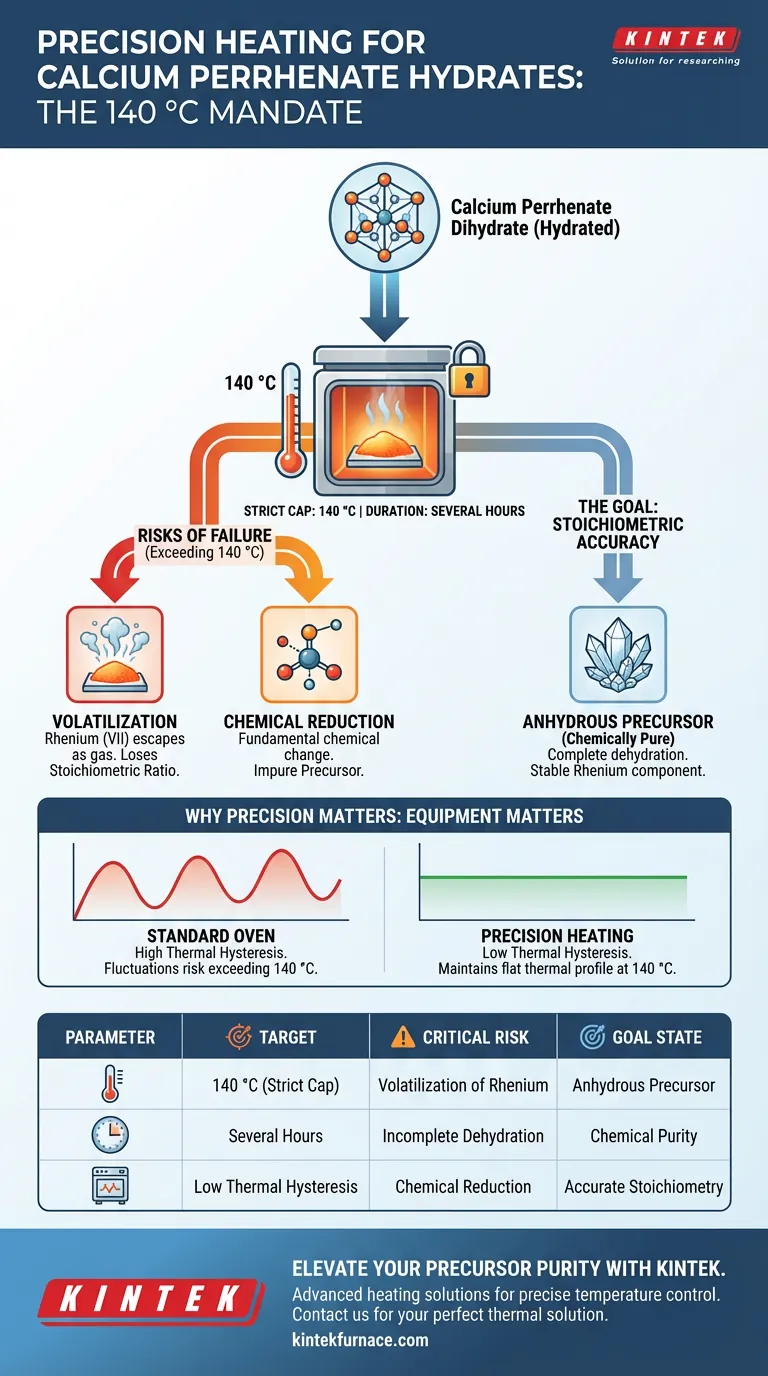
Temperature-controlled heating is critical for successfully converting calcium perrhenate dihydrate into its anhydrous form without compromising the sample's chemical integrity. Maintaining a strict environment at 140 °C allows for the complete removal of crystallization water while specifically preventing the loss or alteration of the Rhenium component.
Precision heating ensures the complete dehydration of calcium perrhenate while protecting the stability of Rhenium (VII). Without this strict control, you risk volatilizing the rhenium or altering the stoichiometric ratio, resulting in an impure precursor.
The Goal: Stoichiometric Accuracy
Removing Crystallization Water
The primary objective of this thermal process is dehydration. You are starting with calcium perrhenate dihydrate, which contains water molecules within its crystal structure.
To create a usable precursor, you must drive off this water completely to achieve an anhydrous state.
Ensuring Chemical Purity
The process requires a stable, constant-temperature environment. Fluctuations in heat can lead to incomplete drying or, conversely, degradation of the material.
Precision equipment ensures that the sample remains chemically pure by holding the temperature at the exact threshold required for dehydration, without exceeding the limits of chemical stability.
Why Strict Control is Mandatory
Preventing Volatilization
The most critical risk in this process is the loss of Rhenium. If the temperature exceeds the safe threshold, Rhenium (VII) is prone to volatilization.
If volatilization occurs, the Rhenium escapes as a gas. This destroys the stoichiometric ratio of your compound, rendering the precursor inaccurate and unusable for precise applications.
Avoiding Chemical Reduction
Beyond simple evaporation, excessive heat can fundamentally change the chemical nature of the compound.
Uncontrolled heating can cause the reduction of Rhenium (VII). To maintain the correct oxidation state and ensure the anhydrous calcium perrhenate is chemically valid, the temperature must not spike above the 140 °C target.
Understanding the Trade-offs
Process Speed vs. Integrity
There is often a desire to increase temperature to accelerate drying times. However, in this context, speed is the enemy of purity.
You cannot compensate for the "several hours" required by simply raising the temperature. The strict cap of 140 °C acts as a safety ceiling; exceeding it to save time will almost certainly result in Rhenium loss or reduction.
Equipment Requirements
Standard laboratory ovens may suffer from thermal hysteresis (fluctuations where the temperature swings above and below the set point).
Because Rhenium (VII) is sensitive to these upper limits, standard equipment may be insufficient. You must use precision laboratory heating equipment capable of maintaining a flat thermal profile to ensure the upper safety limit is never breached.
Optimizing Your Dehydration Process
The success of your preparation depends on balancing total water removal against the thermal sensitivity of your compound.
- If your primary focus is Chemical Purity: Prioritize the upper temperature limit of 140 °C to prevent the reduction or volatilization of Rhenium (VII).
- If your primary focus is Stoichiometric Accuracy: Ensure the duration of heating is sufficient at 140 °C to achieve complete removal of all crystallization water.
By adhering to this strict thermal profile, you ensure the production of a chemically accurate and stable anhydrous precursor.
Summary Table:
| Process Parameter | Target/Requirement | Critical Risk of Failure |
|---|---|---|
| Target Temperature | 140 °C (Strict Cap) | Volatilization of Rhenium (VII) |
| Duration | Several Hours | Incomplete Dehydration |
| Goal State | Anhydrous Precursor | Chemical Reduction / Impurity |
| Equipment Needs | Low Thermal Hysteresis | Inaccurate Stoichiometric Ratio |
Elevate Your Precursor Purity with KINTEK
Precision is non-negotiable when handling sensitive compounds like Rhenium. KINTEK’s advanced heating solutions are engineered to eliminate thermal hysteresis, ensuring your samples never exceed critical stability thresholds.
Backed by expert R&D and world-class manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems. Whether you need standard lab equipment or a high-temperature furnace customized for your unique dehydration protocols, we provide the stability your research demands.
Protect your stoichiometric accuracy today. Contact our specialists to find your perfect thermal solution.
Visual Guide
References
- New calcium perrhenates: synthesis and crystal structures of Ca(ReO<sub>4</sub>)<sub>2</sub> and K<sub>2</sub>Ca<sub>3</sub>(ReO<sub>4</sub>)<sub>8</sub>·4H<sub>2</sub>O. DOI: 10.1515/zkri-2025-0008
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- Why are stainless steel tubes used during the cooling and heat treatment stages of Ti–Nb–Si alloys? Key Cooling Insights
- What role do high-purity graphite crucibles play in Boron Carbide sintering? Optimize Ceramic Purity and Density
- What are the reasons for using high-purity alumina material for the reaction tubes in a Drop Tube Furnace? - Guide
- What are the benefits of sealing SAC305 solder in vacuum quartz tubes? Ensure High-Reliability Alloy Integrity
- How does the selection of a ceramic crucible contribute to the preparation of biomass carbon catalysts? Maximize Purity
- How does a high-precision vacuum pump reduce reaction temperatures in zinc extraction? Optimize Your Energy Efficiency
- What is the critical physical function of a laboratory electric blast drying oven in phosphor gel treatment?
- What is the function of graphite stirring rods in aluminum casting? Achieve Perfect Alloy Homogenization



















