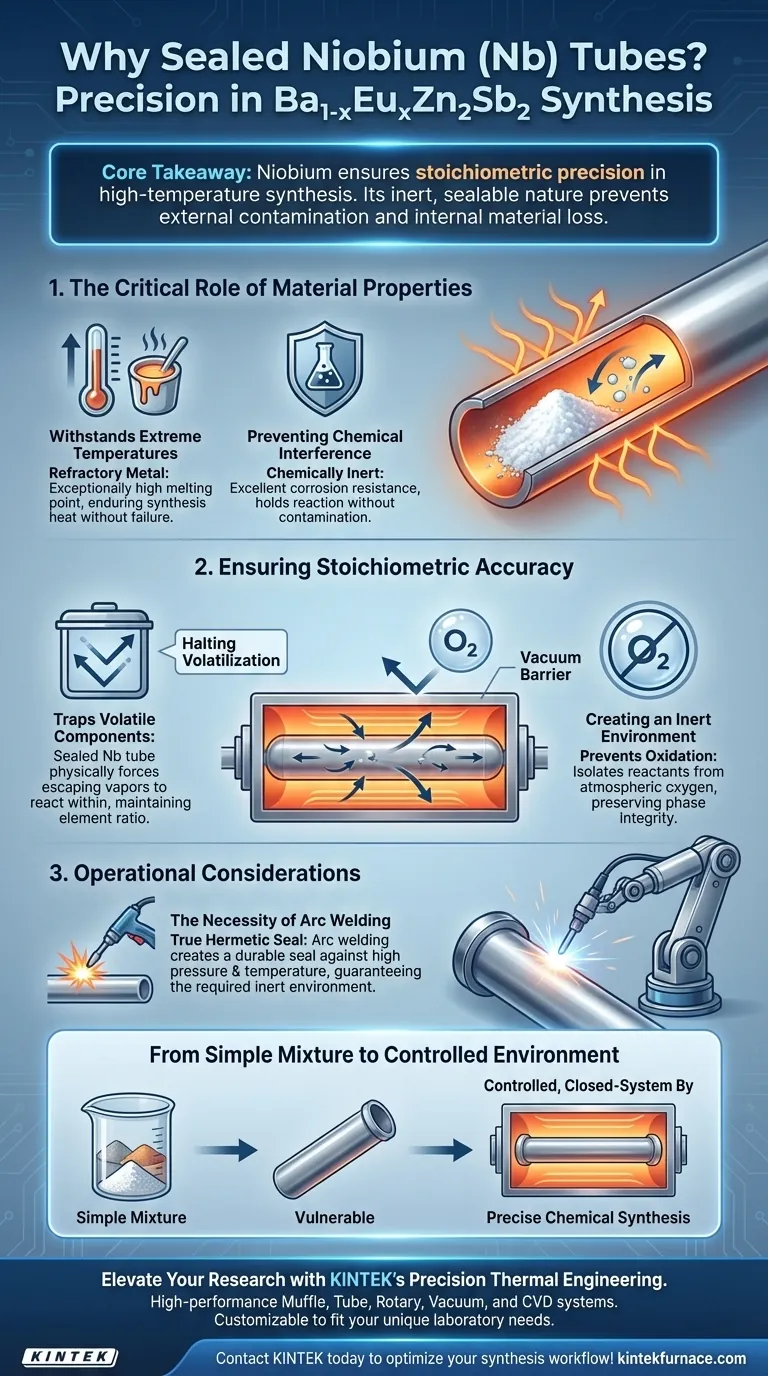
Sealed Niobium (Nb) tubes are chosen as reaction vessels for the synthesis of Ba1-xEuxZn2Sb2 primarily due to their refractory nature and ability to create a hermetically sealed, inert environment. They safeguard the reaction against oxidation and the loss of volatile components, ensuring the final compound matches the intended chemical formula.
Core Takeaway The use of Niobium is a strategic choice to guarantee stoichiometric precision in high-temperature synthesis. By utilizing a vessel that is both chemically inert and capable of being arc-welded shut, researchers prevent external contamination and internal material loss that would otherwise ruin the experiment.

The Critical Role of Material Properties
Withstanding Extreme Temperatures
Niobium is a refractory metal, meaning it possesses an exceptionally high melting point.
This property allows the reaction vessel to endure the extreme heat required for the solid-state synthesis of Ba1-xEuxZn2Sb2. Standard container materials would likely fail or deform under these thermal conditions.
Preventing Chemical Interference
A successful synthesis requires that the container does not become part of the reaction.
Niobium offers excellent corrosion resistance and remains chemically inert relative to the reactants. This ensures that the vessel "holds" the reaction without chemically contaminating the mixture or altering the final product's purity.
Ensuring Stoichiometric Accuracy
Halting Volatilization
At high temperatures, certain raw materials in the mixture may become volatile and attempt to escape as gas.
If these components escape, the ratio of elements changes, ruining the stoichiometric ratio. The sealed Nb tube physically traps these vapors, forcing them to react within the vessel rather than dissipating.
Creating an Inert Environment
Solid-state synthesis is highly sensitive to oxygen.
When sealed, the Niobium tube isolates the reactants from the outside atmosphere. This prevents oxidation of the raw materials, which is critical for maintaining the integrity of the Ba1-xEuxZn2Sb2 phase.
Operational Considerations and Trade-offs
The Necessity of Arc Welding
Unlike simple screw-top or crimped containers, Niobium tubes require arc welding to seal effective.
This process is necessary to create a true hermetic seal that can withstand high pressure and temperature. While this adds a step to the preparation process, it is the only way to guarantee the "inert environment" required for this specific synthesis.
Making the Right Choice for Your Goal
The selection of Niobium is not arbitrary; it is an engineering decision based on the strict requirements of the synthesis process.
- If your primary focus is Compositional Control: Rely on sealed Nb tubes to prevent the volatilization of reactants, ensuring your final product matches your calculated stoichiometry.
- If your primary focus is Phase Purity: Use Niobium to eliminate the risk of oxidation and container-wall reactions that leads to impurities.
By sealing reactants in Niobium, you transition from a simple mixture to a controlled, closed-system environment capable of precise chemical synthesis.
Summary Table:
| Feature | Advantage | Benefit to Synthesis |
|---|---|---|
| High Refractoriness | Withstands extreme synthesis temperatures | Maintains structural integrity without melting. |
| Chemical Inertness | Resists corrosion and reaction with precursors | Ensures high phase purity and zero contamination. |
| Hermetic Sealing | Prevents escape of volatile elements | Guarantees exact stoichiometric precision. |
| Inert Atmosphere | Isolates reactants from oxygen/moisture | Prevents oxidation of sensitive raw materials. |
Elevate Your Research with Precision Thermal Engineering
Achieve unmatched precision in your solid-state synthesis with KINTEK’s high-performance thermal solutions. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all designed to meet the rigorous demands of advanced material science.
Whether you are working with Niobium reaction vessels or specialized alloys, our high-temperature furnaces provide the uniform heating and atmosphere control necessary for superior phase purity and stoichiometric accuracy. All our systems are customizable to fit your unique laboratory needs.
Ready to optimize your synthesis workflow? Contact KINTEK today to discuss your requirements!
Visual Guide
References
- Daewon Shim, Tae‐Soo You. Eu-Substituents-Induced Modifications in the Thermoelectric Properties of the Zintl Phase Ba1-xEuxZn2Sb2 System. DOI: 10.3390/molecules30020310
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- Why must fuel injectors used in high-temperature furnace systems incorporate a cooling function? Prevent Coking Today
- How does heating equipment with magnetic stirring contribute to Fe3O4 synthesis? Achieve Precise Nanoparticle Control
- Why is an external cooling system vital for high-temperature furnace stability? Protect Your Research Integrity
- Why use high-performance insulation bricks in radiant tube simulations? Ensure precision and industrial accuracy.
- What role do quartz tubes play in semiconductor manufacturing? Essential for Purity and High-Temp Processes
- Why is the use of high-vacuum pump groups critical for photothermal catalytic chamber pre-treatment?
- Why is a corundum crucible required for the sintering of manganese ore at 1200 °C? Ensure High-Purity Results
- What is the primary function of a high-alumina powder crucible? Ensure Purity in Maraging Steel Pre-treatment



















