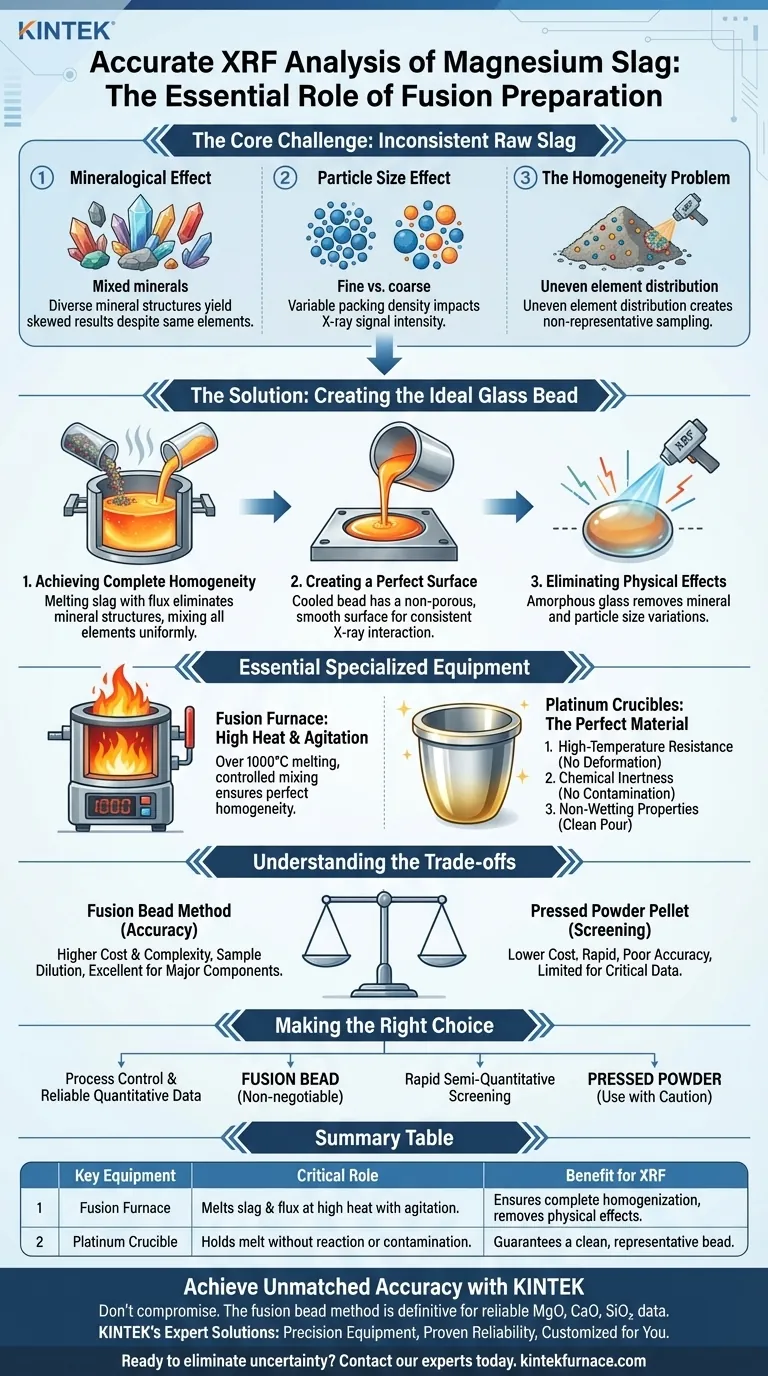
To achieve accurate analysis of magnesium slag, a fusion furnace and platinum crucibles are used to transform the inconsistent, powdered raw material into a perfectly uniform glass disc. This critical preparation step melts the slag with a flux, completely eliminating physical and mineralogical variations in the sample that would otherwise render X-ray fluorescence (XRF) results unreliable.
The core challenge in analyzing materials like magnesium slag is their inherent non-uniformity. The fusion bead method is the definitive solution, ensuring the X-ray beam interacts with a perfectly homogeneous sample, which is the only way to guarantee precise and repeatable chemical measurements.

The Core Challenge: Why Raw Slag is Unsuitable for XRF
Directly analyzing a pressed powder of magnesium slag with XRF leads to inaccurate data. This is due to several physical and chemical inconsistencies within the sample material that interfere with the measurement process.
The Mineralogical Effect
Magnesium slag is not a single chemical compound but a mix of different minerals. Each mineral has a unique crystal structure that interacts with X-rays differently, even if the overall elemental composition is the same. This variation skews the analytical results.
The Particle Size Effect
The size and packing of particles in a pressed powder pellet directly impact X-ray signal intensity. Finer particles can lead to a stronger signal than coarser particles of the exact same material, introducing a significant source of error that cannot be easily corrected.
The Homogeneity Problem
The distribution of elements within the raw slag powder is rarely uniform. The small area analyzed by the XRF beam may not be representative of the entire sample, leading to results that do not reflect the true bulk composition.
How Fusion Creates the Ideal Analytical Sample
The fusion process is designed to systematically eliminate every source of sample-related error by creating an entirely new, ideal material for analysis.
Achieving Complete Homogeneity
The process involves melting the slag with a flux (like sodium tetraborate) at very high temperatures. This completely dissolves the original mineral structures, thoroughly mixing all elements into a uniform molten glass solution.
Creating a Perfect Surface
This molten glass is then cooled in a mold to form a solid bead. The resulting bead has a perfectly flat, smooth, and non-porous surface, which is the ideal geometry for consistent and predictable interaction with the X-ray beam.
Eliminating All Physical Effects
By creating this new, amorphous glass state, the problematic mineralogical and particle size effects are completely removed. The XRF spectrometer is now free to measure the true elemental composition without physical interference.
The Essential Role of Specialized Equipment
Achieving this perfect transformation from a powder to a glass bead requires highly specific tools capable of handling the extreme conditions of the process.
Why a Fusion Furnace?
A specialized fusion furnace is required to provide the intense heat (often over 1000°C) needed to melt the slag and flux. Crucially, these instruments also provide controlled agitation or rocking, which is essential for ensuring the molten mixture is perfectly homogenized before cooling.
Why Platinum Crucibles?
Platinum (often alloyed with gold) is the material of choice for crucibles and molds for three critical reasons:
- High-Temperature Resistance: It has a very high melting point and can easily withstand the fusion process without deforming or failing.
- Chemical Inertness: Platinum does not react with the sample or the flux, preventing any contamination that would compromise the analysis.
- Non-Wetting Properties: The molten glass does not stick to the platinum surface. This allows for a clean, complete pour into the mold, ensuring the final bead is an accurate representation of the entire prepared sample.
Understanding the Trade-offs
While the fusion method is superior for accuracy, it is important to understand its practical implications.
Cost and Complexity
Platinum crucibles and automated fusion furnaces represent a significant investment compared to the simple hydraulic press used for making powder pellets. The process also requires more time and skilled operation.
Sample Dilution
Adding flux to the slag dilutes the sample. This lowers the signal intensity for all elements, which can be a challenge for detecting trace-level components. However, for the major components in slag (e.g., MgO, CaO, SiO₂), this is a necessary and acceptable trade-off for accuracy.
Making the Right Choice for Your Goal
Your analytical goal should dictate your sample preparation method.
- If your primary focus is process control and reliable quantitative data: The fusion bead method is non-negotiable for accurately analyzing the major components of magnesium slag.
- If your primary focus is rapid, semi-quantitative screening: A pressed powder pellet might offer a quick check, but the results must be treated with extreme caution and are not suitable for critical decisions.
Ultimately, the fusion method is the industry standard because it replaces sample uncertainty with analytical certainty.
Summary Table:
| Key Equipment | Critical Role in Fusion | Benefit for XRF Analysis |
|---|---|---|
| Fusion Furnace | Melts slag and flux at high temperatures (>1000°C) with agitation. | Ensures complete sample homogenization, eliminating mineralogical and particle size effects. |
| Platinum Crucible | Holds the melt without reacting or contaminating the sample. | Guarantees a clean, representative bead, free from impurities that would skew results. |
Achieve Unmatched Accuracy in Your Slag Analysis
Don't let sample inconsistency compromise your XRF results. The fusion bead method is the definitive solution for reliable quantitative data on major components like MgO, CaO, and SiO₂.
KINTEK's expert solutions are designed for your success:
- Precision Equipment: Our Muffle, Tube, and high-temperature furnaces are engineered for the exacting demands of fusion preparation.
- Proven Reliability: Backed by expert R&D and manufacturing, our systems ensure repeatable, contamination-free results.
- Customized for You: We tailor our furnaces to meet your lab's unique workflow and sample requirements.
Ready to eliminate analytical uncertainty? Contact our experts today to find the perfect fusion solution for your laboratory.
Visual Guide
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How does a vertical tube furnace achieve precise temperature control? Unlock Superior Thermal Stability for Your Lab
- What is an example of a material prepared using a tube furnace? Master Precise Material Synthesis
- What recent improvements have been made to lab tube furnaces? Unlock Precision, Automation & Safety
- What safety measures are essential when operating a lab tube furnace? A Guide to Preventing Accidents
- How is a high-temperature tube furnace utilized in the synthesis of MoO2/MWCNTs nanocomposites? Precision Guide



















