The aggressive chemical nature of liquid antimony-tellurium alloys dictates the choice of crucible material in high-temperature environments. High-purity graphite or quartz are essential because liquid chalcogenides react destructively with most standard metallic containers. These specialized materials provide the necessary chemical inertness and thermal shock resistance to prevent crucible erosion and safeguard the purity of the melt.
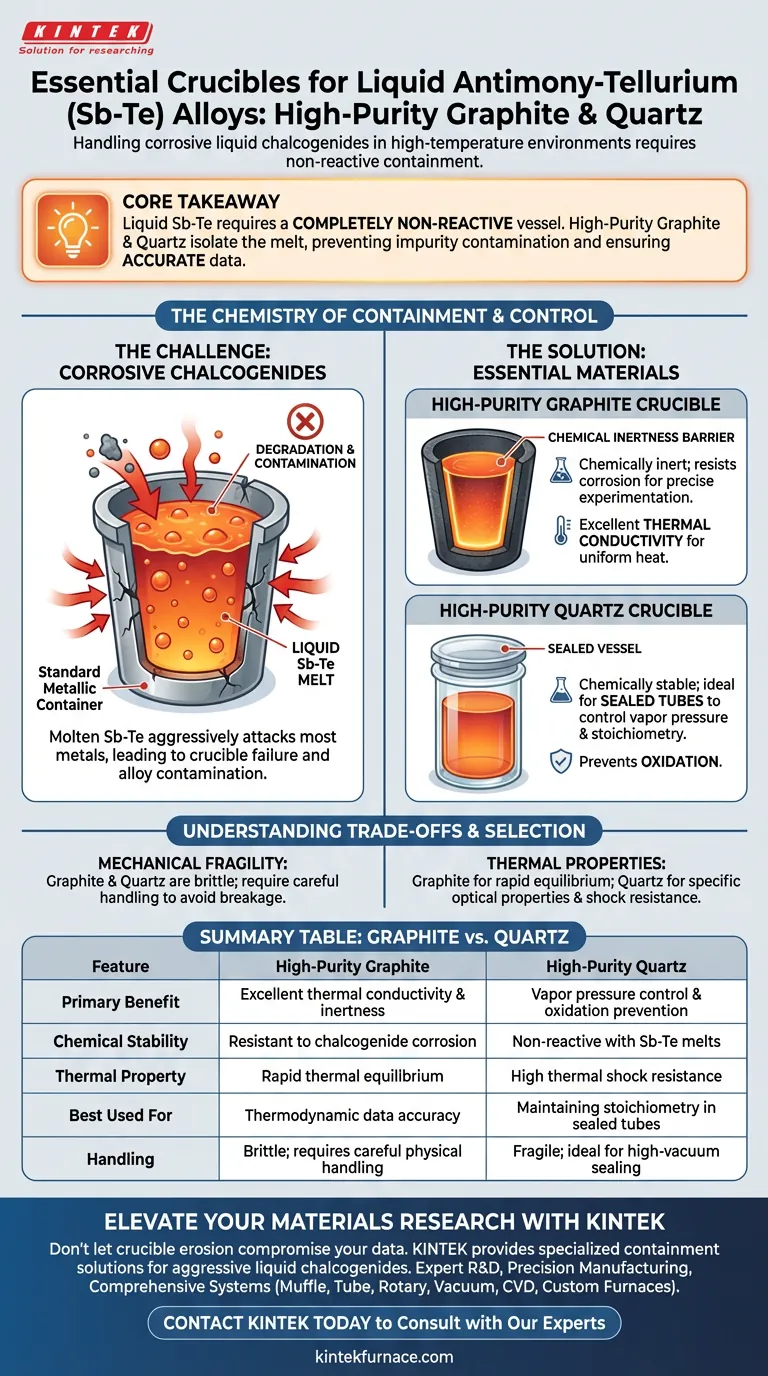
Core Takeaway Handling liquid antimony-tellurium (Sb-Te) requires a containment vessel that is completely non-reactive. High-purity graphite and quartz are the industry standards because they isolate the corrosive melt from the environment, preventing impurity contamination and ensuring the accuracy of thermodynamic data.

The Chemistry of Containment
The Corrosive Nature of Chalcogenides
Liquid chalcogenides, a group that includes antimony and tellurium, possess extreme chemical corrosiveness.
When in a molten state, these elements can aggressively attack and react with most metallic materials.
Using standard metal crucibles would lead to immediate degradation of the vessel and catastrophic contamination of the alloy.
The Necessity of Chemical Inertness
The primary function of the crucible is to act as a neutral barrier.
High-purity graphite and quartz function effectively because they are chemically inert relative to the Sb-Te melt.
This inertness ensures that the crucible does not dissolve into the alloy, preserving the material's integrity for precise experimentation.
Impact on Data Accuracy
For scientific applications, specifically thermodynamic calculations, purity is non-negotiable.
Any reaction between the melt and the crucible introduces impurities that alter the alloy's properties.
By resisting erosion, graphite and quartz ensure that the data derived from the melt reflects the true properties of the alloy, not a contaminated mixture.
Thermal and Environmental Control
Resistance to Thermal Shock
High-temperature environments subject materials to rapid fluctuations in heat.
High-purity graphite and quartz possess superior thermal shock resistance, allowing them to withstand these changes without cracking or failing.
This mechanical stability is as critical as chemical stability during the heating and cooling cycles of the melting process.
Controlling Vapor Pressure and Stoichiometry
When using high-purity quartz as a sealed vessel, it offers advantages beyond simple containment.
Sealed quartz tubes maintain a constant vapor pressure, which is critical for volatile elements like antimony and tellurium.
This confinement ensures precise chemical stoichiometry and overall homogeneity, preventing the loss of active elements during the melt.
Prevention of Oxidation
At elevated temperatures, active elements in the alloy are highly susceptible to oxidation.
Quartz vessels, particularly when sealed or used in high-vacuum environments, create an airtight barrier.
This effectively blocks oxygen, preventing the formation of oxides that would degrade the alloy's quality.
Understanding the Trade-offs
Mechanical Durability vs. Chemical Purity
While quartz and graphite are chemically superior, they lack the ductility of metals.
They are brittle materials that require careful handling to avoid mechanical breakage, distinct from the chemical erosion they resist.
Users must prioritize handling protocols to prevent physical damage to these chemically robust vessels.
Thermal Conductivity Considerations
Graphite offers excellent thermal conductivity, aiding in uniform heat distribution.
Quartz, while chemically stable, has different thermal transfer properties that may affect heating rates.
Selecting between them may depend on whether your process requires rapid thermal equilibrium or specific optical properties (transparency) offered by quartz.
Making the Right Choice for Your Goal
To select the correct containment strategy for your specific application, consider the following:
- If your primary focus is thermodynamic accuracy: Prioritize high-purity graphite to eliminate any risk of metallic contamination affecting your calculations.
- If your primary focus is precise stoichiometry: Utilize sealed high-purity quartz tubes to maintain vapor pressure and prevent the loss of volatile components like antimony.
- If your primary focus is oxidation prevention: Ensure your crucible setup allows for a high-vacuum or airtight seal to protect active elements from the atmosphere.
By aligning your crucible material with the chemical realities of chalcogenides, you convert a potential point of failure into a guarantee of experimental integrity.
Summary Table:
| Feature | High-Purity Graphite | High-Purity Quartz |
|---|---|---|
| Primary Benefit | Excellent thermal conductivity & inertness | Vapor pressure control & oxidation prevention |
| Chemical Stability | Resistant to chalcogenide corrosion | Non-reactive with Sb-Te melts |
| Thermal Property | Rapid thermal equilibrium | High thermal shock resistance |
| Best Used For | Thermodynamic data accuracy | Maintaining stoichiometry in sealed tubes |
| Handling | Brittle; requires careful physical handling | Fragile; ideal for high-vacuum sealing |
Elevate Your Materials Research with KINTEK
Don't let crucible erosion compromise your thermodynamic data or contaminate your high-purity alloys. KINTEK provides the specialized containment solutions you need to master aggressive liquid chalcogenides.
Backed by expert R&D and precision manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with customizable high-temp lab furnaces designed for your unique experimental requirements.
Ready to ensure the purity and stoichiometry of your next melt?
Contact KINTEK Today to Consult with Our Experts
Visual Guide
References
- В. Н. Володин, Azamat Tulegenov. Thermodynamics of Liquid Alloys and Vapor–Liquid Equilibrium in the Antimony–Tellurium System. DOI: 10.1007/s12540-023-01564-x
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1200℃ Muffle Oven Furnace for Laboratory
People Also Ask
- How does an electromechanical vibrator assist in fuel feeding? Enhance Coal and Biomass Combustion Stability
- What is the importance of using a cooling jacketed sampling probe? Ensure Accurate Drop Tube Furnace Data
- What role do graphite molds play in the Spark Plasma Sintering (SPS)? Enhance Alumina Composite Performance
- What role does a Molybdenum Boat play in ZTO thin film deposition? Master Thermal Evaporation Success
- Why is a MgO crucible preferred for VCD? Achieve 3ppm Purity in High-Temperature Metallurgy
- What function do the piping and butterfly valve components serve in a multi-kiln carbonization system? Maximize Control
- What is the critical role of a mechanical vacuum pump in WS2 gas sensor prep? Ensure High Purity & Performance
- What role do graphite molds play in graphite flake alignment? Engineered Precision for High Thermal Conductivity



















