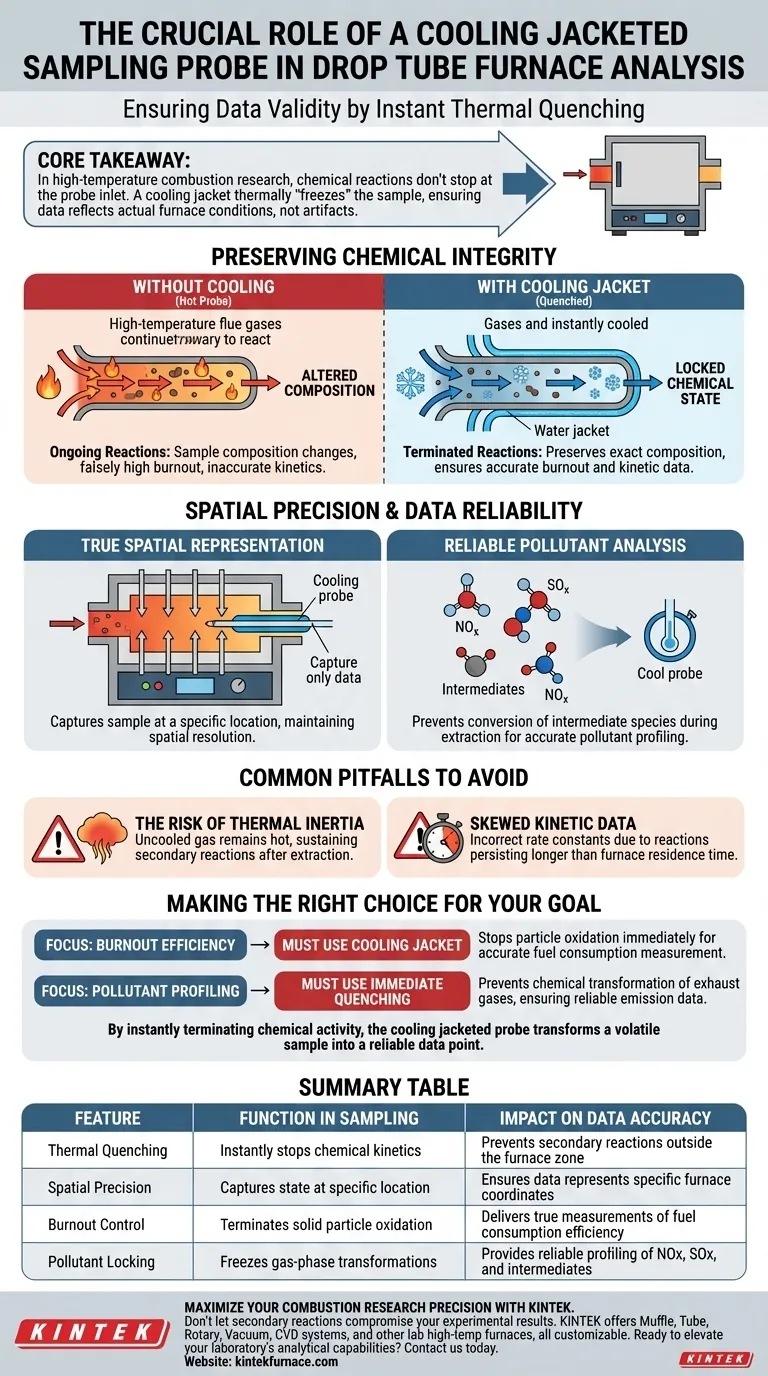
A cooling jacketed sampling probe is critical for ensuring the validity of combustion data collected from a Drop Tube Furnace. Its primary function is to provide immediate cooling to flue gases and solid particles as they are extracted, instantly "quenching" the sample to preserve its state.
Core Takeaway In high-temperature combustion research, chemical reactions do not stop simply because a sample enters a probe. A cooling jacket is required to thermally "freeze" the sample, ensuring that the data reflects the actual furnace conditions rather than artifacts created by reactions continuing inside the sampling line.

Preserving Chemical Integrity
Terminating Reactions Immediately
The environment inside a Drop Tube Furnace involves complex, high-velocity chemical kinetics. If a sample is extracted without rapid cooling, ongoing chemical reactions will continue as the gas travels through the probe.
This allows the sample's composition to change between the extraction point and the analyzer. The cooling jacket prevents this by dropping the temperature of the sample instantly, effectively locking its chemical state.
Accurate Burnout Evaluation
To measure burnout efficiency, researchers must know exactly how much fuel has been consumed at a specific point in the furnace.
If the solid particles continue to react (oxidize) inside a hot sampling probe, the final analysis will falsely indicate a higher burnout efficiency than actually occurred in the furnace. The cooling jacket eliminates this error source.
Spatial Precision and Data Reliability
True Spatial Representation
Drop Tube Furnaces are often used to map combustion behavior over distance or time. A cooling jacketed probe ensures that the collected sample represents the chemical composition at a specific spatial location.
Without this mechanism, the sample becomes an average of the extraction point and the reaction history within the probe, destroying the spatial resolution of your data.
Reliable Pollutant Analysis
The formation of pollutants, such as Nitrogen Oxides (NOx) or Sulfur Oxides (SOx), is highly temperature-dependent.
To accurately study the formation of pollutants, you must capture the gas composition exactly as it exists in the high-temperature zone. Rapid quenching prevents the conversion of intermediate species into different pollutants during the extraction process.
Common Pitfalls to Avoid
The Risk of Thermal Inertia
A common mistake in sampling is underestimating the thermal inertia of the extracted gas. Without active cooling, the gas remains hot enough to sustain secondary reactions for milliseconds or seconds after extraction.
Skewed Kinetic Data
If you attempt to derive reaction kinetics from uncooled samples, your rate constants will be incorrect. The "time" variable in your calculation becomes distorted because reactions persisted longer than the residence time in the furnace suggests.
Making the Right Choice for Your Goal
When configuring your Drop Tube Furnace experiments, the use of a cooling jacketed probe is determined by your need for chemical precision.
- If your primary focus is Burnout Efficiency: You must use a cooling jacket to stop particle oxidation immediately upon extraction, ensuring the remaining mass accurately reflects the furnace state.
- If your primary focus is Pollutant Profiling: You need immediate quenching to prevent the chemical transformation of exhaust gases as they cool naturally, which would skew emission data.
By instantly terminating chemical activity, the cooling jacketed probe transforms a volatile sample into a reliable data point.
Summary Table:
| Feature | Function in Sampling | Impact on Data Accuracy |
|---|---|---|
| Thermal Quenching | Instantly stops chemical kinetics | Prevents secondary reactions outside the furnace zone |
| Spatial Precision | Captures state at specific location | Ensures data represents specific furnace coordinates |
| Burnout Control | Terminates solid particle oxidation | Delivers true measurements of fuel consumption efficiency |
| Pollutant Locking | Freezes gas-phase transformations | Provides reliable profiling of NOx, SOx, and intermediates |
Maximize Your Combustion Research Precision with KINTEK
Don’t let secondary reactions compromise your experimental results. At KINTEK, we understand the critical role of thermal quenching in high-temperature analysis.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, CVD systems, and other lab high-temp furnaces, all customizable for your unique research needs. Whether you are mapping pollutant formation or measuring burnout efficiency, our precision-engineered systems provide the stability and control you require.
Ready to elevate your laboratory’s analytical capabilities? Contact us today to discuss your custom furnace requirements and see how our expertise can drive your innovation.
Visual Guide
References
- Garikai T. Marangwanda, Daniel M. Madyira. Evaluating Combustion Ignition, Burnout, Stability, and Intensity of Coal–Biomass Blends Within a Drop Tube Furnace Through Modelling. DOI: 10.3390/en18061322
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- Why are high-purity alumina tubes and crucibles preferred for high-temperature smelting? Ensure Maximum Sample Purity
- How do a precision hydraulic press and high-strength stainless steel molds facilitate boron carbide green body forming?
- Why is a mass flow controller essential in the tracer method? Precision Data for Pyrolysis Gas Flow
- How do graphite molds in SPS affect maraging steel? Managing Carbon Diffusion for Precise Sintering Results
- How does a high-precision heating stage contribute to the drying and crystallization of FAPbBr3 nanosheets?
- What is the role of a mechanical vacuum pump in the preparation of FeAl alloys? Achieve 10⁻² Pa for Pure Synthesis
- Why is rhenium selected as a material for sample capsules? Key Benefits for High-Temperature Experimental Success
- Why use a fusion furnace and platinum crucibles for XRF analysis of magnesium slag? Ensure Accurate Results



















