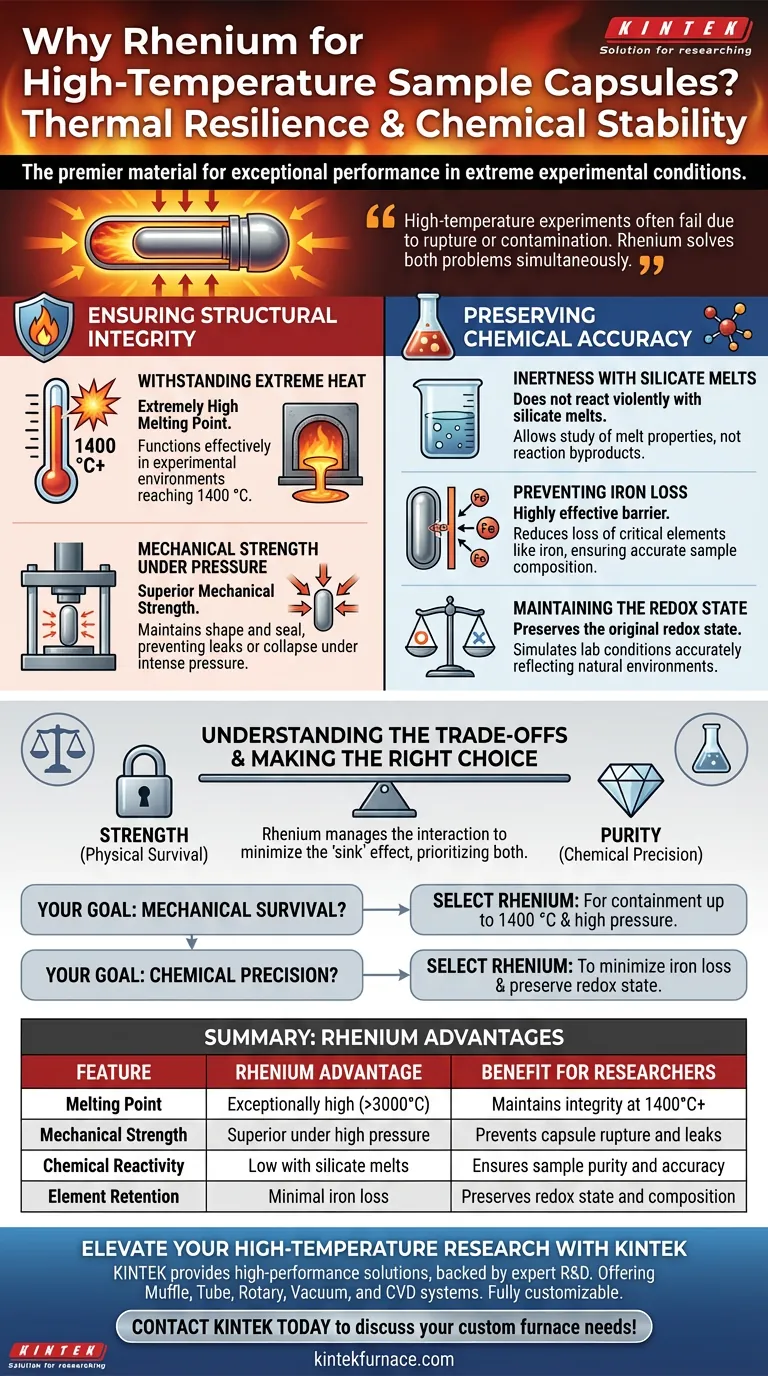
Rhenium is the material of choice for high-temperature sample capsules primarily due to its exceptional combination of thermal resilience and chemical stability. It is specifically selected to maintain structural integrity at temperatures as high as 1400 °C while subjected to high-pressure conditions, ensuring the experiment remains contained without failure.
High-temperature experiments often fail due to capsule rupture or chemical contamination of the sample. Rhenium is utilized because it solves both problems simultaneously: it withstands extreme physical stress and prevents the migration of critical elements, ensuring the chemical data you collect is accurate.

Ensuring Structural Integrity
Withstanding Extreme Heat
The primary prerequisite for these experiments is a container that will not melt or deform excessively.
Rhenium possesses an extremely high melting point. This allows it to function effectively in experimental environments reaching 1400 °C, a range where many other metals would fail.
Mechanical Strength Under Pressure
Temperature is rarely the only stressor; these experiments often involve significant pressure.
Rhenium offers superior mechanical strength, ensuring the capsule maintains its shape and seal. This robustness prevents the sample from leaking or the capsule from collapsing under the intense crush of the experimental apparatus.
Preserving Chemical Accuracy
Inertness with Silicate Melts
A major challenge in geochemistry is the potential for the capsule to react with the sample.
Rhenium is selected because it does not react violently with silicate melts. This relative inertness allows researchers to study the properties of the melt itself, rather than the byproducts of a reaction between the melt and the container.
Preventing Iron Loss
One of the most common sources of experimental error in high-temperature petrology is the loss of iron from the sample into the capsule walls.
Rhenium acts as a highly effective barrier or lining that reduces the loss of critical elements like iron. By keeping the iron within the sample, the experiment yields more accurate data regarding the sample's composition.
Maintaining the Redox State
The oxidation state of a system (redox) is highly sensitive to changes in chemical composition.
By preventing the loss of iron, Rhenium helps maintain the original redox state of the experimental system. This ensures that the conditions simulated in the lab accurately reflect the natural conditions being modeled.
Understanding the Trade-offs
Balancing Strength and Purity
While Rhenium is robust, the decision to use it often comes down to the specific chemical interactions acceptable for your study.
The primary "trade-off" Rhenium manages is mitigating the interaction between container and sample. While no material is perfectly inert, Rhenium minimizes the "sink" effect where the capsule absorbs sample elements. Using an inferior material would result in significant chemical alteration, rendering the experimental data invalid.
Making the Right Choice for Your Goal
When designing your high-temperature assembly, consider your specific analytical needs:
- If your primary focus is mechanical survival: Select Rhenium to ensure containment at temperatures up to 1400 °C under high pressure.
- If your primary focus is chemical precision: Select Rhenium to minimize iron loss and preserve the precise redox state of silicate melts.
By selecting Rhenium, you prioritize both the physical survival of your experiment and the chemical validity of your results.
Summary Table:
| Feature | Rhenium Advantage | Benefit for Researchers |
|---|---|---|
| Melting Point | Exceptionally high (>3000°C) | Maintains integrity at 1400°C+ |
| Mechanical Strength | Superior under high pressure | Prevents capsule rupture and leaks |
| Chemical Reactivity | Low with silicate melts | Ensures sample purity and accuracy |
| Element Retention | Minimal iron loss | Preserves redox state and composition |
Elevate Your High-Temperature Research with KINTEK
Precision in geochemistry and material science demands equipment that can withstand the most extreme conditions without compromising data integrity. KINTEK provides the high-performance solutions your lab needs to succeed.
Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, along with other specialized lab high-temp furnaces—all fully customizable to meet your unique experimental parameters. Whether you are managing complex redox states or requiring capsules that survive intense pressure, our team is ready to support your goals.
Ready to optimize your thermal processes? Contact KINTEK today to discuss your custom furnace and high-temperature equipment needs!
Visual Guide
References
- Wanying Wang, Yuan Li. Redox control of the partitioning of platinum and palladium into magmatic sulfide liquids. DOI: 10.1038/s43247-024-01366-y
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What roles do high-purity graphite molds perform in A357 sintering? Enhancing Aluminum Matrix Composite Performance
- What key roles do high-purity graphite molds play in SPS? Powering High-Entropy Carbide Synthesis
- Why is ceramic refractory material used in the freeboard area of a gasification reactor? Enhance Your Syngas Purity
- Why is a platinum (Pt) crucible selected as the reaction vessel? Ensure Precision in High-Temp Molten Salt Research
- Why is selecting the right laboratory furnace important for ceramic sintering? Ensure Precise Control for Superior Ceramic Properties
- How are constant temperature water baths and drying ovens utilized to verify bonding quality? Master EN 314-1 Testing
- What are the technical requirements for the Quartz Boat used as a precursor container in the CVD growth of 2D In2Se3?
- What role does a planetary ball mill play in Al-Cr-Cu-Fe-Mn-Ni alloy prep? Master Mechanical Alloying Efficiency














