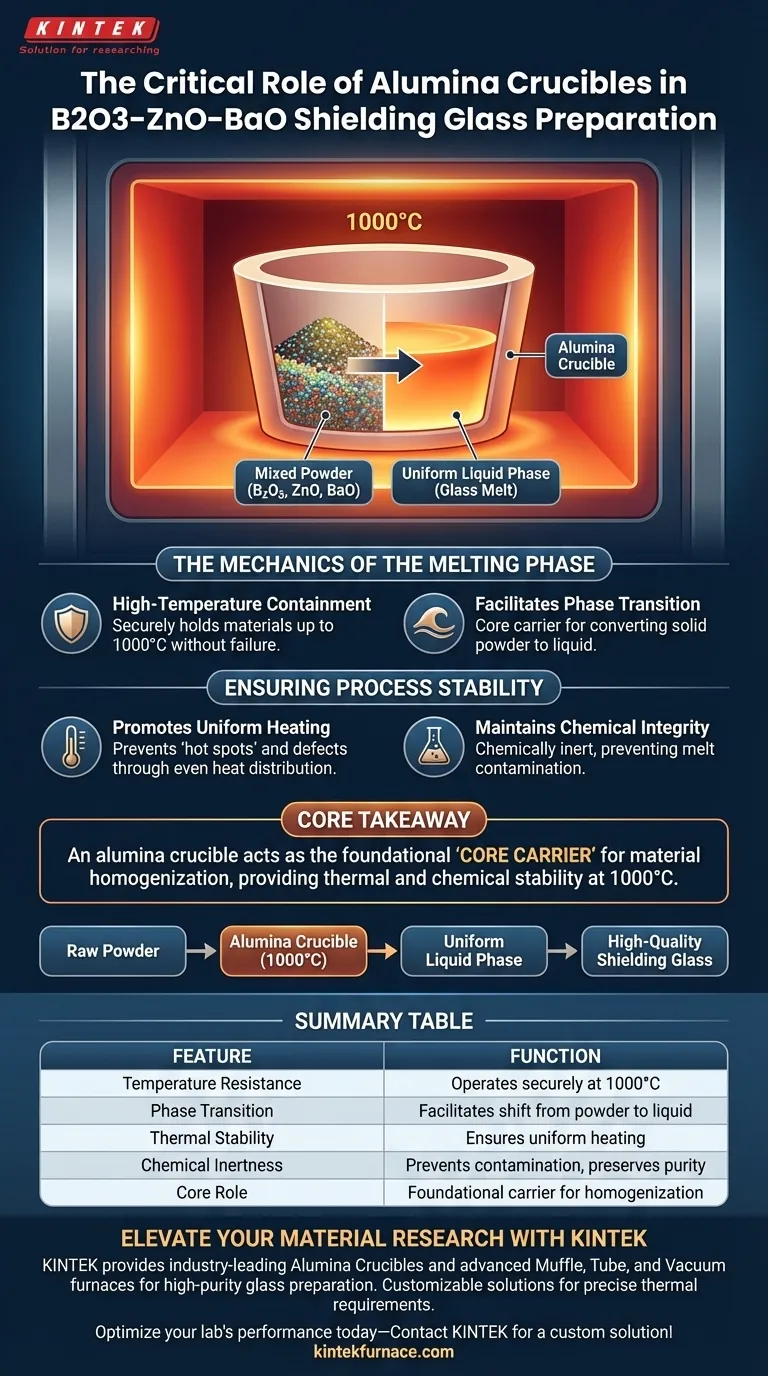
The primary function of an alumina crucible in this context is to serve as a high-temperature resistant vessel that facilitates the transition of B2O3–ZnO–BaO raw materials from a mixed powder into a uniform liquid phase. It is specifically utilized to withstand temperatures up to 1000°C, ensuring the materials are heated evenly without compromising the chemical integrity of the resulting glass melt.
Core Takeaway An alumina crucible acts as the foundational "core carrier" for glass preparation, providing the necessary thermal and chemical stability to process raw powders at 1000°C. Its main role is to ensure a homogenous melt while preventing contamination or container failure during the intense heating phase.

The Mechanics of the Melting Phase
Containing High Temperatures
The preparation of B2O3–ZnO–BaO shielding glass requires subjecting raw materials to intense heat. The alumina crucible is selected primarily for its ability to function as a high-temperature resistant container.
It must securely hold the mixed powders as the environment ramps up to 1000°C. Without this thermal resistance, the containment would fail before the melting process could complete.
Facilitating Phase Transition
The ultimate goal of the melting phase is to convert solid powder into a liquid. The crucible serves as the core carrier for this physical transformation.
By providing a stable environment, it allows the mixed powders to transition smoothly into a uniform liquid phase. This step is critical for ensuring the final glass has consistent properties throughout.
Ensuring Process Stability
Promoting Uniform Heating
Thermal stability goes beyond simply not melting; it involves how the material conducts and manages heat. The alumina crucible ensures that the raw materials inside are heated uniformly.
Uniform heating is essential to prevent "hot spots" or uneven melting, which could lead to structural defects in the shielding glass.
Maintaining Chemical Integrity
During the melting process, the container must not react with its contents. The alumina crucible is relied upon for its chemical stability regarding the melt.
It holds the B2O3–ZnO–BaO mixture without leaching contaminants or reacting adversely, thereby preserving the purity and chemical composition of the glass.
Critical Operational Requirements
Adhering to Temperature Limits
While alumina is robust, the process is specifically defined around a 1000°C operational parameter.
The effectiveness of the crucible is tied to this temperature range. Exceeding this temperature significantly without verifying the crucible's specific grade could risk the stability of the container or the melt.
The Necessity of Uniformity
The crucible is not just a bucket; it is a tool for homogenization. If the crucible fails to maintain thermal stability, the uniformity of the liquid phase is compromised.
A failure in this function results in a glass product that may lack the consistent shielding properties required for its end application.
Making the Right Choice for Your Goal
To ensure the successful preparation of B2O3–ZnO–BaO shielding glass, you must align your equipment choices with the thermal and chemical demands of the process.
- If your primary focus is Melt Homogeneity: Ensure your heating profile ramps effectively to 1000°C to leverage the crucible's ability to distribute heat uniformly.
- If your primary focus is Material Purity: Rely on the chemical stability of the alumina to prevent interaction between the vessel wall and the B2O3–ZnO–BaO melt.
The alumina crucible is the linchpin of the melting phase, bridging the gap between raw powder and a stable, high-quality liquid glass.
Summary Table:
| Feature | Function in B2O3–ZnO–BaO Preparation |
|---|---|
| Temperature Resistance | Operates securely at 1000°C without structural failure |
| Phase Transition | Facilitates the shift from raw powder to uniform liquid phase |
| Thermal Stability | Ensures uniform heating to prevent structural defects in glass |
| Chemical Inertness | Prevents contamination and preserves melt purity |
| Core Role | Acts as the foundational carrier for material homogenization |
Elevate Your Material Research with KINTEK
Achieving the perfect glass melt requires more than just high temperatures—it demands precision-engineered equipment. KINTEK provides industry-leading Alumina Crucibles and advanced Muffle, Tube, and Vacuum furnace systems specifically designed for high-purity glass preparation and material science.
Our expert R&D and manufacturing team ensures that every system is customizable to your unique thermal requirements, delivering the stability and uniformity your research depends on. Optimize your lab's performance today—Contact KINTEK for a custom solution!
Visual Guide
References
- Mohamed Elsafi, Taha A. Hanafy. Experimental study of different oxides in B2O3–ZnO–BaO glass system for gamma-ray shielding. DOI: 10.1038/s41598-025-85230-9
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What are the advantages of using a single-mode microwave generator? Precision Heating for Metal Recovery
- What is the temperature range for Laboratory Type Furnaces? Find Your Ideal Heat Solution
- What is polycrystalline mullite/alumina wool (PCW) and where is it used? Discover High-Temp Insulation Solutions
- Why are silicon carbide crucibles selected for C95800 aluminum bronze? Ensure Purity & Efficiency
- What role does a high-precision mass flow controller play in assessing the gas selectivity of Gallium Sulfide sensors?
- What role do vacuum-sealed high-purity silica ampoules play in phase equilibrium experiments? Enhance Sample Integrity
- What are the advantages of using open corundum crucibles for thermal analysis? Accurate Biomass & Coal Data
- What is the purpose of applying Boron Nitride (BN) to graphite molds in Mg3Sb2 VHP? Ensure Purity & Easy Demolding



















