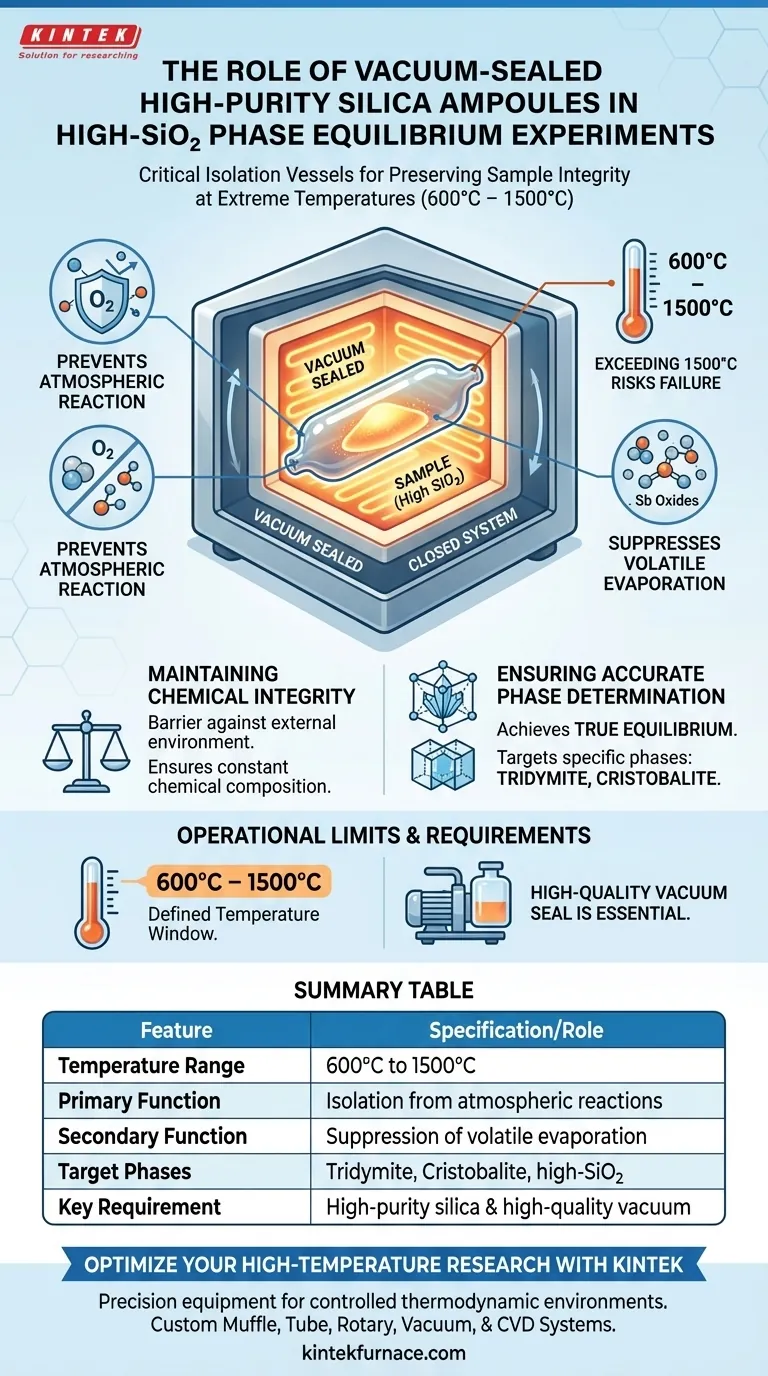
Vacuum-sealed high-purity silica ampoules serve as critical isolation vessels in phase equilibrium experiments, functioning specifically to preserve sample integrity at extreme temperatures between 600°C and 1500°C. Their primary role is to create a closed system that prevents the sample from reacting with the surrounding atmosphere while simultaneously suppressing the evaporation of volatile components.
By maintaining a sealed environment under vacuum, these ampoules ensure that the chemical composition remains constant, allowing for the accurate determination of solid-liquid equilibrium within specific silica phase regions like tridymite and cristobalite.

Maintaining Chemical Integrity at High Temperatures
Prevention of Atmospheric Interaction
The fundamental function of the silica ampoule is to act as a barrier.
By enclosing the sample, the ampoule prevents any chemical reaction between the experimental materials and the external environment, such as furnace gases or oxygen.
Suppression of Volatile Component Loss
In systems containing volatile elements, such as antimony oxides, maintaining composition is difficult due to evaporation.
The vacuum encapsulation technique suppresses the vaporization of these components.
This ensures that the stoichiometry of the sample remains unchanged throughout the heating process.
Ensuring Accurate Phase Determination
Achieving True Equilibrium
Accurate phase diagrams depend on the system remaining closed.
These ampoules guarantee that the observed solid-liquid equilibrium reflects the initial mixture design rather than a composition altered by mass loss.
Targeting Specific Silica Phases
The ampoules are specifically effective for studying high-SiO2 content regions.
They provide the stable environment necessary to isolate and identify phases such as silica, tridymite, and cristobalite.
Understanding the Operational Limits
Temperature Constraints
While robust, these ampoules have a defined operating window.
They are effective strictly within the 600°C to 1500°C range.
Exceeding the upper limit of 1500°C risks compromising the structural integrity of the silica glass itself.
The Necessity of Vacuum
The effectiveness of the ampoule is entirely dependent on the quality of the seal.
Without a proper vacuum seal, the suppression of volatile loss is compromised, rendering the equilibrium data invalid.
Making the Right Choice for Your Experiment
To ensure the validity of your phase equilibrium data, apply the following guidelines:
- If your primary focus is preserving stoichiometry: Utilize vacuum encapsulation to trap volatile components like antimony oxides that would otherwise escape.
- If your primary focus is high-temperature phase mapping: Ensure your experimental design stays within the 600-1500°C limit to accurately characterize tridymite or cristobalite regions without ampoule failure.
Success in these experiments relies on treating the ampoule not just as a container, but as an active component in controlling the thermodynamic environment.
Summary Table:
| Feature | Specification/Role |
|---|---|
| Temperature Range | 600°C to 1500°C |
| Primary Function | Isolation of samples from atmospheric reactions |
| Secondary Function | Suppression of volatile component evaporation (e.g., Antimony oxides) |
| Target Phases | Tridymite, Cristobalite, and high-SiO2 regions |
| Key Requirement | High-purity silica & high-quality vacuum sealing |
Optimize Your High-Temperature Research with KINTEK
Precision in phase equilibrium experiments demands more than just a container; it requires a controlled thermodynamic environment. KINTEK provides the specialized equipment needed to support your most sensitive material studies.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as other lab high-temp furnaces—all fully customizable to your unique experimental needs. Whether you are mapping silica phases or suppressing volatile mass loss, our systems provide the stability and temperature control your data depends on.
Ready to elevate your lab's accuracy? Contact our experts today to find the perfect thermal solution for your research.
Visual Guide
References
- Hamed Abdeyazdan, Evgueni Jak. Phase equilibria in the CuO <sub>0.5</sub> –SbO <sub>1.5</sub> –SiO <sub>2</sub> system. DOI: 10.1111/jace.70123
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Magnesium Extraction and Purification Condensing Tube Furnace
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
People Also Ask
- Why is the integration of a K-type thermocouple and a data logger necessary for Vanadis 60 steel? Unlock Precision.
- What is the function of high-vacuum quartz sealing tubes in TiCo1-xCrxSb heat treatment? Ensure Alloy Purity
- Why are high-purity MgO crucibles used for PbO oxidation? Essential Chemical Inertness for Master Slags
- Why are metal wire mesh trays preferred for thin-layer drying? Boost Efficiency and Accuracy in Your Lab
- Why Is a High-Precision Heating and Stirring Platform Necessary for ZnO Sol-Gel Synthesis? Achieve Perfect Nanoparticles
- Why is the use of casting flux necessary during the melting of aluminum-based alloys? Protect Your Chemical Integrity
- What are the benefits of a vacuum chamber? Achieve Unmatched Process Control and Purity
- What are the typical applications of a circulating water vacuum pump? Essential for Lab Efficiency and Cost Savings













