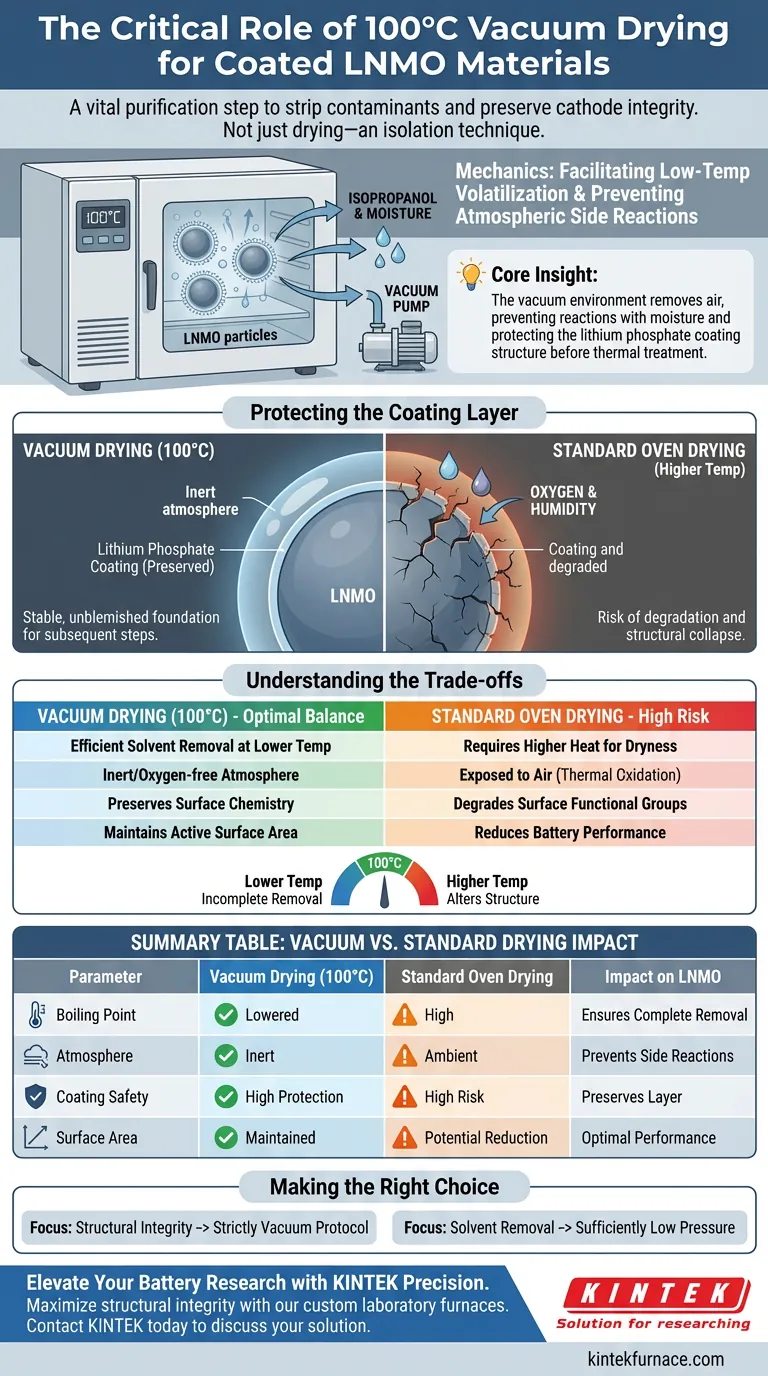
The application of vacuum drying at 100°C is a critical purification step designed to strip away volatile contaminants without compromising the chemical stability of your cathode material. This specific protocol ensures the complete removal of isopropanol solvent and residual moisture while simultaneously isolating the reactive Lithium Nickel Manganese Oxide (LNMO) surface from atmospheric interference.
Core Insight: The vacuum environment is not merely about drying; it is an isolation technique. By removing air, you prevent side reactions between the LNMO and atmospheric moisture, thereby protecting the structural integrity of the delicate lithium phosphate coating layer before final thermal treatment.
The Mechanics of Vacuum Drying LNMO
Facilitating Low-Temperature Volatilization
The primary function of the laboratory vacuum drying oven is to reduce internal pressure. This physical change lowers the boiling point of solvents like isopropanol.
By operating under reduced pressure, you allow these solvents to volatilize efficiently at 100°C. This ensures thorough removal of liquid agents used during the coating process without requiring excessive heat that could damage the material.
Preventing Atmospheric Side Reactions
Standard drying ovens expose materials to ambient air, which contains oxygen and humidity. At elevated temperatures, LNMO is highly susceptible to reacting with these atmospheric impurities.
The vacuum environment eliminates this variable entirely. It creates an inert space where the material can dry without engaging in unwanted chemical changes, specifically preserving the material's surface chemistry.
Protecting the Coating Layer
The ultimate goal of this drying phase is to preserve the lithium phosphate coating layer. This coating is often newly formed and chemically vulnerable.
If exposed to moisture or high-temperature oxidation during the drying phase, this layer could degrade or suffer structural collapse. Vacuum drying establishes a stable, unblemished foundation necessary for the subsequent curing and thermal treatment steps.
Understanding the Trade-offs
The Risk of Standard Oven Drying
Attempting to replicate this process in a standard, non-vacuum oven is a common point of failure. Without reduced pressure, higher temperatures are often required to achieve the same level of dryness.
This increased thermal stress, combined with exposure to air, frequently leads to the thermal oxidation of the material surface. This can reduce the active specific surface area and degrade surface functional groups, ultimately lowering battery performance.
Balancing Temperature and Pressure
While 100°C is the standard, deviations can be detrimental. Lower temperatures may result in incomplete solvent removal, leading to defects during the final firing.
Conversely, significantly higher temperatures—even under vacuum—risk altering the crystalline structure of the LNMO before the coating is properly set. The 100°C vacuum protocol represents the optimal balance between efficient solvent removal and material preservation.
Making the Right Choice for Your Goal
When establishing your synthesis protocol, consider your specific purity and structural requirements:
- If your primary focus is Structural Integrity: Adhere strictly to the vacuum protocol to prevent side reactions that compromise the lithium phosphate coating.
- If your primary focus is Solvent Removal: Ensure the vacuum pressure is sufficiently low to fully volatilize isopropanol at 100°C, preventing porosity defects in later stages.
Correctly executing this drying step effectively "locks in" the quality of your precursor material, setting the stage for a high-performance final product.
Summary Table:
| Parameter | Vacuum Drying (100°C) | Standard Oven Drying | Impact on LNMO |
|---|---|---|---|
| Boiling Point | Lowered (Efficient volatilization) | High (Requires more heat) | Ensures complete solvent removal |
| Atmosphere | Inert / Oxygen-free | Ambient Air / Humidity | Prevents surface side reactions |
| Coating Safety | High Protection | High Risk of Degradation | Preserves lithium phosphate layer |
| Surface Area | Maintained | Potential Reduction | Ensures optimal battery performance |
Elevate Your Battery Research with KINTEK Precision
Maximize the structural integrity of your cathode materials with laboratory equipment designed for sensitive thermal processing. Backed by expert R&D and world-class manufacturing, KINTEK offers high-performance Vacuum, Muffle, Tube, Rotary, and CVD systems—all fully customizable to meet your unique laboratory needs. Whether you are drying delicate LNMO precursors or performing high-temp sintering, our furnaces provide the stability and control your research demands.
Ready to optimize your synthesis workflow? Contact KINTEK today to discuss your custom furnace solution with our technical experts.
Visual Guide
References
- So Young Choi, Hyun Deog Yoo. Synthesis and Electrochemical Properties of the Li3PO4-Coated LiNi0.5Mn1.5O4 Cathode Materials for High-Voltage Lithium-Ion Batteries. DOI: 10.3390/en18133387
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- Why is maintaining a high vacuum environment essential during the liquid phase sintering of Fe-Cu composites?
- How does a vacuum furnace improve the mechanical properties of workpieces? Enhance Strength and Durability
- How does a vacuum annealing furnace reduce pollution? Achieve Cleaner Metal Processing with Zero Oxidation
- What operational advantages does a vacuum heat treatment furnace offer? Achieve Superior Metallurgical Quality and Precision
- In which industries is the vacuum carburizing furnace commonly used? Essential for Aerospace and High-Performance Machinery
- What factors should be considered when choosing between argon and nitrogen for vacuum furnace applications? Optimize Your Heat Treatment Process
- What are the advantages of graphite furnace? Achieve Unmatched High-Temperature Performance
- What are some applications of graphite materials in vacuum furnace processing? Discover Key Uses and Benefits



















