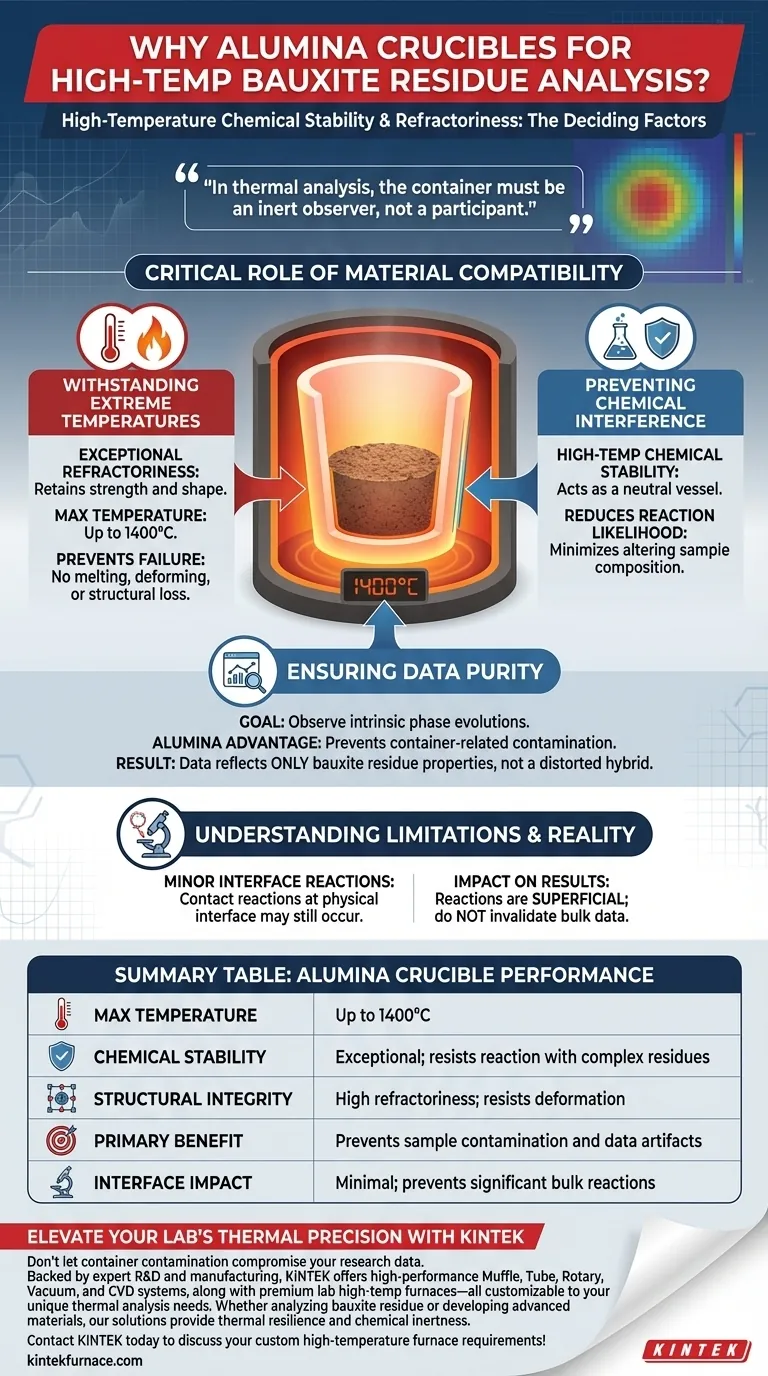
High-temperature chemical stability is the deciding factor. An alumina crucible is required for bauxite residue experiments primarily due to its exceptional refractoriness, which allows it to withstand temperatures as high as 1400 degrees Celsius without failing. Crucially, this material choice prevents the container from reacting significantly with the sample, ensuring that the thermal analysis data remains pure and free from contamination artifacts.
In thermal analysis, the container must be an inert observer, not a participant. Alumina provides the necessary barrier to ensure that observed phase evolutions are intrinsic to the bauxite residue, rather than the result of container degradation.

The Critical Role of Material Compatibility
Withstanding Extreme Temperatures
Bauxite residue analysis often requires subjecting samples to intense thermal environments. The primary requirement for the sample container is refractoriness—the ability to retain strength and shape under high heat.
Alumina crucibles are specifically selected because they maintain their structural integrity up to 1400 degrees Celsius. This prevents the vessel from melting, deforming, or failing during the heating cycle.
Preventing Chemical Interference
At elevated temperatures, materials become more reactive. A container with lower chemical stability could bond or react with the complex chemical makeup of bauxite residue.
Alumina offers exceptional high-temperature chemical stability. It acts as a neutral vessel, drastically reducing the likelihood of the container altering the sample's chemical composition during the experiment.
Ensuring Data Purity
The goal of thermal analysis is to observe how the phases of the bauxite residue evolve. If the container reacts with the sample, it introduces foreign elements or compounds into the mix.
Using alumina prevents container-related contamination. This ensures that the data collected reflects only the properties of the bauxite residue, rather than a distorted hybrid of sample and container.
Understanding the Limitations
The Reality of Interface Reactions
While alumina is highly stable, it is not infinitely impervious. The primary reference notes that minor contact reactions may still occur at the physical interface between the crucible and the sample.
Impact on Results
These interface reactions are generally superficial. While they technically exist, the use of alumina keeps them from becoming significant reactions that would invalidate the bulk data. Researchers can proceed with confidence, knowing the core observations remain accurate.
Ensuring Experimental Success
To obtain reliable data in high-temperature bauxite residue studies, the choice of crucible is as important as the sample preparation.
- If your primary focus is general phase evolution: Rely on alumina to prevent significant chemical interactions and structural failure up to 1400 degrees Celsius.
- If your primary focus is trace interface analysis: Acknowledge that while alumina prevents bulk contamination, minor reactions at the direct contact surface are a known physical possibility.
Alumina provides the essential balance of thermal resilience and chemical inertness required to turn raw experimental heat into actionable scientific insight.
Summary Table:
| Feature | Alumina Crucible Performance |
|---|---|
| Max Temperature | Up to 1400°C |
| Chemical Stability | Exceptional; resists reaction with complex residues |
| Structural Integrity | High refractoriness; resists deformation |
| Primary Benefit | Prevents sample contamination and data artifacts |
| Interface Impact | Minimal; prevents significant bulk reactions |
Elevate Your Lab's Thermal Precision with KINTEK
Don't let container contamination compromise your research data. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, along with premium lab high-temp furnaces—all customizable to your unique thermal analysis needs. Whether you are analyzing bauxite residue or developing advanced materials, our solutions provide the thermal resilience and chemical inertness your experiments demand.
Contact KINTEK today to discuss your custom high-temperature furnace requirements!
Visual Guide
References
- Dali Hariswijaya, Jafar Safarian. Studying the Sintering Behavior of H2-Reduced Bauxite Residue Pellets Using High-Temperature Thermal Analysis. DOI: 10.3390/ma18102378
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What is the purpose of using quartz vacuum encapsulation? Optimize La(Fe,Si)13-based Magnetocaloric Alloys
- Why use a fusion furnace and platinum crucibles for XRF analysis of magnesium slag? Ensure Accurate Results
- Why is a stainless steel closed-end tube required for controlled atmospheric experiments? Ensure Precise Material Purity
- What roles do the high-purity graphite crucible and lid play in PVT AlN growth? Optimize Your Crystal Production
- What is the purpose of using a PID controller to drive a heating furnace? Master Thermal Kinetics Precision
- How do Mass Flow Controllers (MFC) contribute to the repeatability of In2Se3 synthesis? Master CVD Process Stability
- Why is vacuum quartz tube sealing technology required in the synthesis of ZnPS3 crystals? Ensuring Chemical Purity
- Why are quartz tubes indispensable in advanced technologies? Unlock Purity and Performance



















