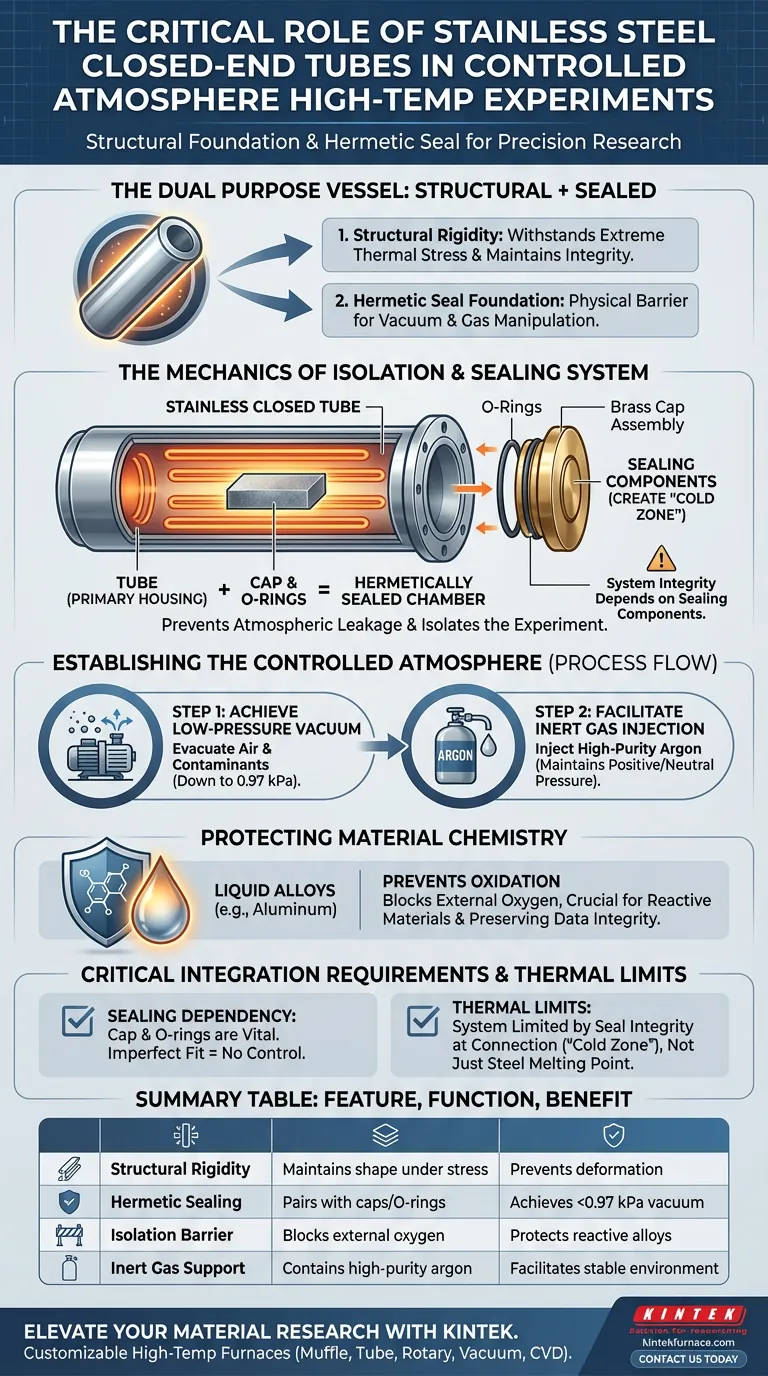
A stainless steel closed-end tube acts as the primary containment vessel required to isolate a high-temperature experiment from the surrounding environment. It serves a dual purpose: providing the structural rigidity necessary to withstand extreme thermal stress and creating a physical barrier that houses the experimental setup.
To establish a controlled atmosphere, you must have a hermetically sealed chamber capable of resisting external pressure. The stainless steel tube provides the mechanical foundation for this seal, enabling the precise manipulation of vacuum levels and gas composition to protect sensitive materials.

The Mechanics of Isolation
Structural Integrity Under Heat
High-temperature experiments place immense physical stress on containment vessels. The stainless steel tube functions as the primary external housing, offering the structural support needed to maintain the setup's shape and integrity when subjected to intense thermal processing.
Creating the Sealed Chamber
The tube does not work in isolation; it functions as half of a sealing system. When integrated with a brass cap and O-rings, the open end of the tube is closed off, transforming the physical housing into a sealed chamber.
Preventing Atmospheric Leakage
This combination of the steel body and the cap assembly is critical for preventing the ingress of outside air. Without this robust physical seal, the pressure differences required for the experiment would cause immediate leaks, rendering the atmospheric control impossible.
Establishing the Controlled Atmosphere
Achieving Low-Pressure Vacuum
Once sealed, the stainless steel tube allows for the evacuation of air. The system can achieve a low-pressure vacuum environment as low as 0.97 kPa. This step removes existing air and contaminants from the chamber before heating begins.
Facilitating Inert Gas Injection
After the vacuum is established, the tube acts as the vessel for high-purity argon injection. The rigid steel walls contain this inert gas, maintaining the specific positive pressure or neutral environment required for the experiment.
Protecting Material Chemistry
Preventing Oxidation
The ultimate goal of this setup is chemical preservation. By physically blocking external oxygen and holding the argon atmosphere, the tube effectively prevents oxidation.
Specific Application: Liquid Alloys
This is particularly vital for processing reactive materials, such as liquid aluminum alloys. Without the stainless steel tube's isolation capabilities, these alloys would react with oxygen at high temperatures, compromising the experimental data and material properties.
Critical Integration Requirements
The Dependency on Sealing Components
While the stainless steel tube handles the heat and structure, its effectiveness is entirely dependent on the brass cap and O-rings. If these components degrade or are improperly fitted, the steel tube serves only as a structural support and loses its ability to control the atmosphere.
Thermal Limits of the System
It is important to recognize that while the tube withstands high heat, the sealing mechanism (O-rings) creates a "cold zone" requirement at the connection point. The system is limited not just by the melting point of the steel, but by the integrity of the seals at the tube's opening.
Making the Right Choice for Your Goal
To ensure your high-temperature setup functions correctly, assess your specific requirements:
- If your primary focus is Structural Safety: Ensure the stainless steel tube is rated for the specific temperatures of your experiment to prevent deformation.
- If your primary focus is Chemical Purity: Prioritize the quality of the O-ring and brass cap integration to ensure the vacuum can hold at 0.97 kPa without leakage.
The stainless steel tube is the indispensable "body" of your experiment, converting a standard heating process into a precise, contamination-free scientific procedure.
Summary Table:
| Feature | Function in Atmospheric Control | Benefit |
|---|---|---|
| Structural Rigidity | Maintains shape under intense thermal stress | Prevents vessel deformation |
| Hermetic Sealing | Pairs with brass caps/O-rings for vacuum seals | Achieves vacuum levels down to 0.97 kPa |
| Isolation Barrier | Blocks ingress of external oxygen and contaminants | Protects reactive liquid alloys from oxidation |
| Inert Gas Support | Contains high-purity argon under pressure | Facilitates stable, non-reactive environments |
Elevate Your Material Research with KINTEK
Precise atmospheric control is the backbone of high-temperature success. Backed by expert R&D and manufacturing, KINTEK offers a wide range of Muffle, Tube, Rotary, Vacuum, and CVD systems—all customizable to your unique experimental needs. Whether you are processing liquid alloys or advanced ceramics, our high-temp furnaces provide the structural integrity and sealing precision your research demands.
Ready to optimize your lab's thermal processing? Contact us today to discuss your custom furnace requirements!
Visual Guide
References
- Aleksandar M. Mitrašinović, Milinko Radosavljević. Modeling of Impurities Evaporation Reaction Order in Aluminum Alloys by the Parametric Fitting of the Logistic Function. DOI: 10.3390/ma17030728
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- Why use a blast drying oven at 80°C for 24h for palm kernel shells? Optimize Biochar Yield & Efficiency
- Why is a high-performance vacuum pump system necessary for AlV55 alloys? Ensure Aerospace-Grade Purity and Precision
- What is the specific significance of using high-purity corundum crucibles in oxidation weight gain experiments?
- What are the primary functions of a quartz tube reactor? Enhance Hydrogen Production and Induction Efficiency
- Why are high-purity alumina crucibles utilized for CsV3Sb5 crystal growth? Ensure Purity in Self-Flux Synthesis
- What are the key mechanical properties of alumina tubes? Uncover High-Strength, Wear-Resistant Solutions
- What role does a heat-resistant steel retort play in sintering? Mastering Isolation and Pressure for High-Purity Results
- How does an in-situ reaction chamber in HTXRD facilitate BiFeO3 synthesis study? Mapping Real-Time Phase Evolution



















