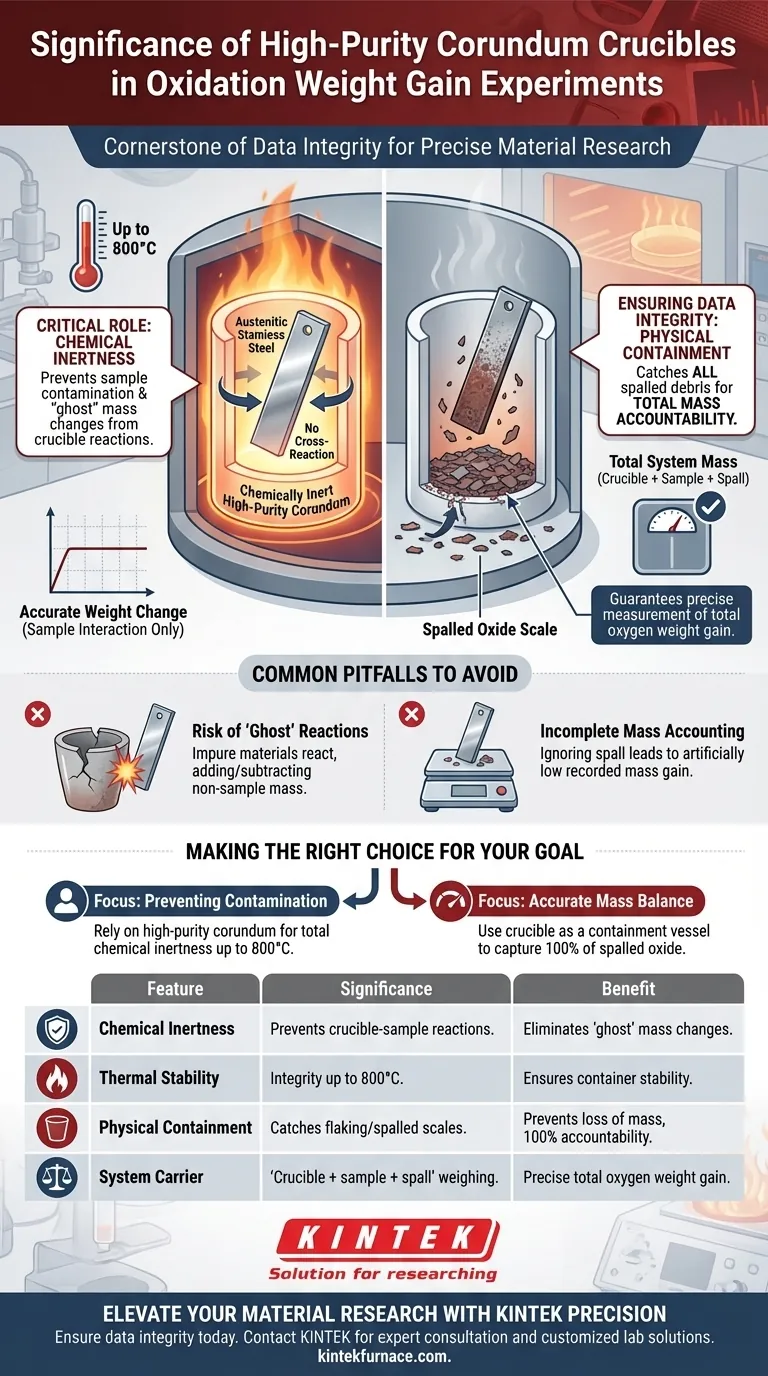
High-purity corundum crucibles serve as the cornerstone of data integrity in oxidation weight gain experiments. Their specific significance lies in a dual function: providing a chemically inert environment that prevents cross-reaction with samples like austenitic stainless steel at temperatures up to 800°C, and acting as a physical catchment system for spalled oxide scales to ensure total mass accountability.
Core Takeaway In static intermittent oxidation methods, the validity of weight gain data relies on accounting for every particle of mass. High-purity corundum crucibles ensure accuracy by preventing chemical interference from the container itself while physically collecting oxide flakes that detach from the sample.

The Critical Role of Chemical Inertness
Preventing Cross-Reactions
The primary significance of using high-purity corundum is its chemical inertness. In high-temperature environments, the vessel itself becomes a variable.
Corundum prevents the crucible from reacting with the sample material. This is explicitly verified for austenitic stainless steel, ensuring that the metal remains isolated from the container's influence.
Stability at 800°C
Oxidation experiments often require sustained exposure to extreme heat. High-purity corundum offers excellent high-temperature stability.
It maintains its structural and chemical integrity up to 800°C. This ensures that no foreign mass—such as degradation byproducts from the crucible—is introduced to the sample during the heating process.
Ensuring Data Integrity via Physical Containment
Addressing Oxide Spalling
During oxidation, the oxide scale formed on the steel surface often cracks and flakes off. This phenomenon, known as spalling, creates a major risk for data loss.
If these flakes fall away and are not weighed, the recorded mass gain will be artificially low. This leads to incorrect conclusions regarding the material's oxidation resistance.
The Crucible as a Carrier
In static intermittent oxidation methods, the corundum crucible acts as a sample carrier. It physically contains both the primary sample and any debris that separates from it.
By catching the spalled oxide scale, the crucible allows the analytical balance to measure the total system mass. This guarantees that the data reflects the true extent of oxidation, rather than just the oxide that managed to stay attached to the metal.
Common Pitfalls to Avoid
The Risk of "Ghost" Reactions
Using crucibles of lower purity or different material composition can introduce experimental error. If the container is not inert, it may react with the steel or the atmosphere.
This reaction adds or subtracts mass that is unrelated to the sample's oxidation. High-purity corundum effectively eliminates this variable, ensuring the weight change observed is solely due to the sample's interaction with oxygen.
Incomplete Mass Accounting
A common error in weight gain experiments is weighing only the sample coupon after heating. This ignores the spalled material.
The significance of the crucible is that it mandates a methodology where the crucible + sample + spall are treated as a single unit for weighing. Neglecting this function renders the data regarding spalling behaviors invalid.
Making the Right Choice for Your Goal
When designing high-temperature oxidation experiments, the choice of crucible material dictates the reliability of your results.
- If your primary focus is preventing contamination: Rely on high-purity corundum to maintain total chemical inertness with stainless steel samples up to 800°C.
- If your primary focus is accurate mass balance: Use the crucible as a containment vessel to capture 100% of spalled oxide scales for precise analytical weighing.
By utilizing high-purity corundum, you transform the crucible from a simple container into an essential instrument for experimental precision.
Summary Table:
| Feature | Significance in Oxidation Experiments | Benefit to Data Accuracy |
|---|---|---|
| Chemical Inertness | Prevents reactions between crucible and sample (e.g., stainless steel). | Eliminates "ghost" mass changes from cross-reactions. |
| Thermal Stability | Maintains integrity at temperatures up to 800°C. | Ensures container stability during long-term heating cycles. |
| Physical Containment | Acts as a catchment system for flaking/spalled oxide scales. | Prevents loss of mass, ensuring 100% accountability. |
| System Carrier | Allows for "crucible + sample + spall" unit weighing. | Guarantees precise measurement of total oxygen weight gain. |
Elevate Your Material Research with KINTEK Precision
Precision in oxidation studies begins with the right environment. KINTEK provides high-performance laboratory solutions designed for the most demanding thermal experiments. Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized high-purity labware tailored for researchers and industrial manufacturers.
Whether you need to maintain absolute chemical inertness or require a custom furnace solution for unique high-temperature needs, our team is ready to support your goals.
Ensure your data integrity today—Contact KINTEK for expert consultation and customized lab solutions.
Visual Guide
References
- Yaoyao Fiona Zhao, Changrong Li. Effect of V content on high temperature oxidation resistance of S30403 austenitic stainless steel. DOI: 10.1038/s41598-025-17971-6
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What is the primary function of high-purity graphite crucibles? Ensure Superior Purity in Aluminum Alloy Melting
- Why are high-purity quartz tubes and quartz boats preferred for plastic pyrolysis? Ensure Precise, Pure Results
- Why are alumina crucibles used for CoNb2O6 synthesis? Ensure High-Purity Ceramic Powder Production
- What process challenges are addressed by vacuum filtration equipment during the construction of CsPbBr3@CA-SiO2 films?
- How does a laboratory blast drying oven facilitate the treatment of Au/ZnO/In2O3 precursor precipitates? Key Benefits
- What is the purpose of using specialized vacuum glass tubes for sampling? Ensure KR Stirring Chemical Integrity
- Why Is a High-Precision Heating and Stirring Platform Necessary for ZnO Sol-Gel Synthesis? Achieve Perfect Nanoparticles
- What are the advantages of using a Type B thermocouple for 1600°C slag reduction? Precision in Ultra-High Heat



















