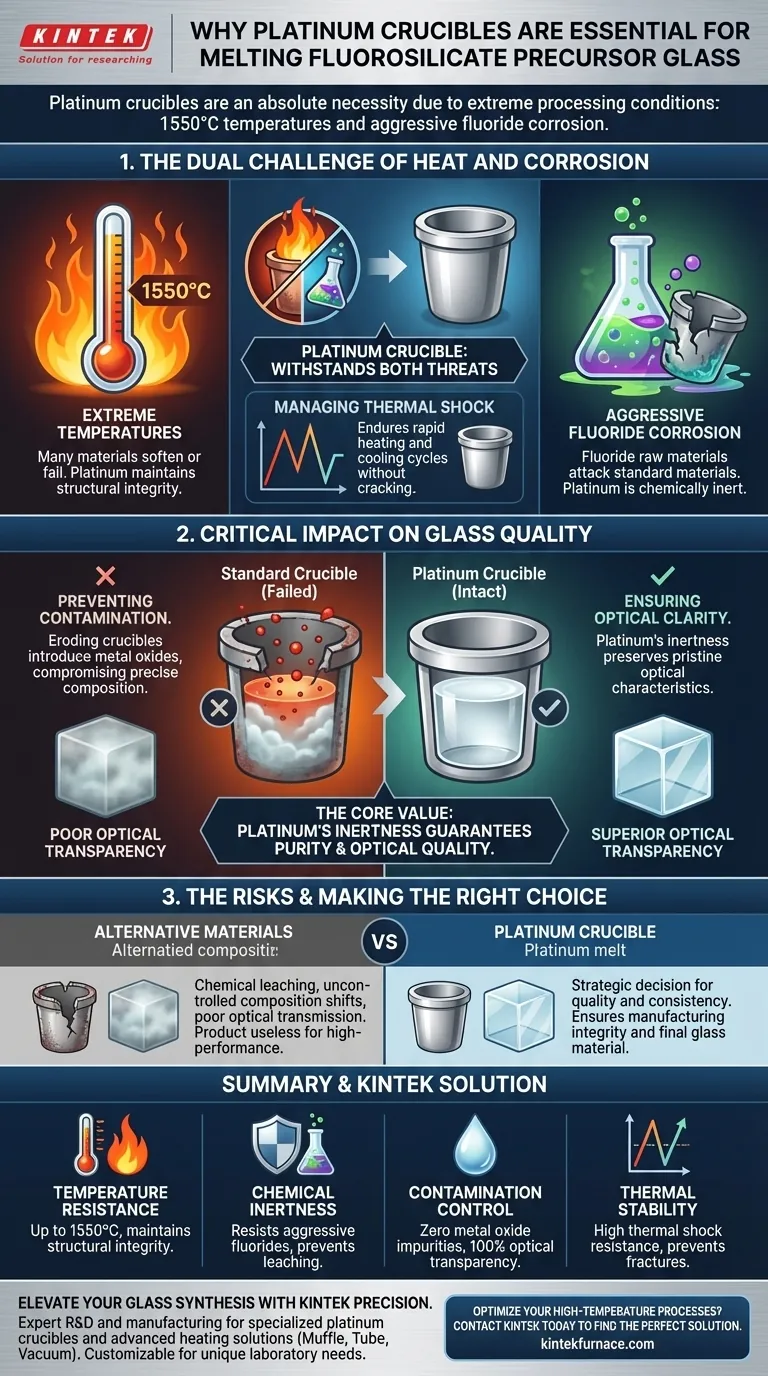
A platinum crucible is an absolute necessity for the melting of fluorosilicate precursor glass due to the extreme severity of the processing environment. The specific combination of melting temperatures reaching 1550°C and the aggressive corrosivity of fluoride raw materials creates conditions that would rapidly degrade standard laboratory equipment.
The core value of platinum lies in its chemical inertness under extreme stress. By withstanding fluoride corrosion at 1550°C without reacting, platinum prevents the leaching of metal oxide impurities, thereby guaranteeing the optical transparency and precise composition essential for the final glass product.

The Dual Challenge of Heat and Corrosion
The production of fluorosilicate precursor glass presents a hostile environment for manufacturing equipment. A platinum crucible is required to address two simultaneous threats to vessel integrity.
Withstanding Extreme Temperatures
The melting process requires temperatures up to 1550°C.
At this thermal threshold, many standard crucible materials soften, deform, or suffer structural failure. Platinum retains its structural integrity, allowing it to contain the melt safely and effectively throughout the heating cycle.
Resisting Chemical Attack
Fluoride raw materials are notoriously corrosive, particularly in a molten state.
Standard ceramic or lower-grade metal crucibles would be chemically attacked by the fluorides. Platinum possesses exceptional chemical inertness, rendering it immune to this specific type of erosion even at peak processing temperatures.
Managing Thermal Shock
The melting process involves significant temperature fluctuations.
Platinum offers superior thermal shock resistance, allowing the crucible to endure rapid heating and cooling cycles without cracking or fracturing.
Critical Impact on Glass Quality
The choice of crucible is not merely about vessel survival; it is directly linked to the quality of the glass produced.
Preventing Contamination
The primary risk in this process is the introduction of impurities into the melt.
If a crucible erodes, it introduces metal oxides and other contaminants into the glass mixture. Platinum’s resistance to erosion eliminates this vector of contamination, ensuring the chemical composition remains precise.
Ensuring Optical Clarity
For fluorosilicate glass, optical performance is often the defining metric.
Impurities introduced by a degrading crucible would compromise the optical transparency of the glass. By remaining inert, the platinum crucible preserves the pristine optical characteristics required for the final application.
The Risks of Material Compromise
When selecting equipment for this process, it is vital to understand the trade-offs of attempting to use alternative materials.
The Cost of Impurity
While platinum is a significant capital investment, the alternative is a compromised product.
Substituting platinum with a less noble material will almost invariably result in chemical leaching. This leads to batches of glass with uncontrolled composition shifts and poor optical transmission, effectively rendering the product useless for high-performance applications.
Making the Right Choice for Your Goal
The selection of a platinum crucible is a strategic decision to prioritize quality and consistency over upfront equipment costs.
- If your primary focus is Optical Transparency: You must use platinum to prevent the introduction of haze-inducing metal oxides.
- If your primary focus is Process Stability: You must use platinum to withstand the combined stress of 1550°C heat and fluoride corrosion without vessel failure.
By using platinum, you ensure the integrity of both the manufacturing process and the final glass material.
Summary Table:
| Feature | Requirement for Fluorosilicate Glass | Why Platinum is Essential |
|---|---|---|
| Temperature Resistance | Up to 1550°C | Maintains structural integrity without softening or deforming. |
| Chemical Inertness | Resistance to aggressive fluorides | Prevents corrosive attack and chemical leaching into the melt. |
| Contamination Control | Zero metal oxide impurities | Non-reactive surface ensures 100% optical transparency. |
| Thermal Stability | Rapid heating/cooling cycles | High thermal shock resistance prevents cracking or fracturing. |
Elevate Your Glass Synthesis with KINTEK Precision
Don't let crucible degradation compromise your high-performance materials. Backed by expert R&D and manufacturing, KINTEK offers specialized platinum crucibles and advanced heating solutions—including Muffle, Tube, and Vacuum systems—all customizable for your unique laboratory needs. Whether you are melting fluorosilicates or developing next-generation glass, our equipment ensures the chemical purity and process stability your research demands.
Ready to optimize your high-temperature processes? Contact KINTEK today to find the perfect solution for your lab.
Visual Guide
References
- Zhigang Gao, Guoping Dong. Robust low threshold full-color upconversion lasing in rare-earth activated nanocrystal-in-glass microcavity. DOI: 10.1038/s41377-024-01671-3
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What are the key properties of effective heating elements? Optimize Your Heat Generation for Efficiency and Longevity
- What is silicon carbide (SiC) and why is it used for heating elements? Unlock High-Temp Efficiency
- How does the Joule heating process work in high-temperature heating elements? Unlock Efficient Heat Generation for Your Lab
- What makes 99.6% high-purity alumina tubes stable under extreme conditions? Discover the Key to Unmatched Thermal and Chemical Resilience
- How do ceramic heating elements reduce maintenance costs compared to metal alternatives? Lower TCO with Durable Ceramic Heaters
- What are the advantages of using platinum/rhodium alloys as heating elements? Unmatched High-Temp Stability & Longevity
- What are silicon carbide (SiC) heating elements made of? A Guide to Extreme-Temperature Performance
- How are silicon carbide heating elements used in chemical processing? Enhance High-Temp Corrosion Resistance



















