The remarkable stability of 99.6% high-purity alumina tubes stems from a combination of their fundamental atomic structure and the near-total absence of impurities. The aluminum and oxygen atoms form exceptionally strong chemical bonds in a dense crystal lattice, providing inherent thermal and physical resilience. The high purity ensures that this intrinsic strength is not compromised by weaker materials that would otherwise cause failure at extreme temperatures.
The core reason for this stability is not a single feature, but a principle: high purity preserves the exceptional intrinsic strength of the alumina crystal structure. Weak points in most ceramics come from impurities, and by minimizing them, the material can perform closer to its theoretical limits.
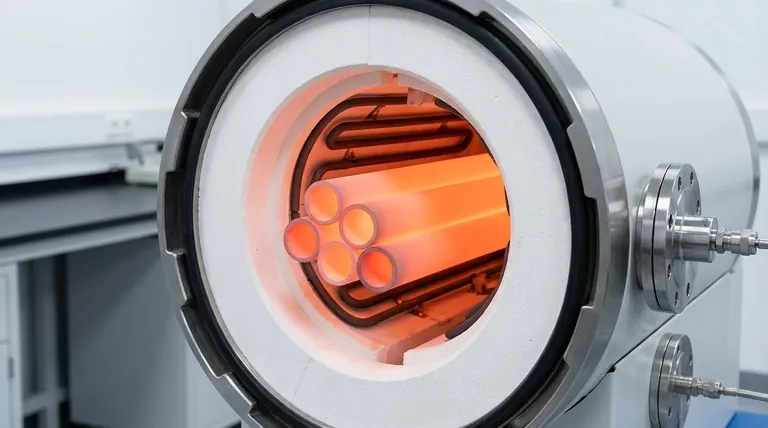
The Foundation of Stability: The Alumina Crystal Structure
The properties of a material are dictated by how its atoms are arranged and bonded. For high-purity alumina, this foundation is exceptionally robust.
What is Alumina (Al₂O₃)?
Alumina is a ceramic compound of aluminum (Al) and oxygen (O). In its most stable, high-performance form, known as corundum (α-alumina), it arranges itself into a hexagonal crystal structure that is both dense and highly ordered.
The Strength of Ionic-Covalent Bonds
The bonds holding the aluminum and oxygen atoms together are a powerful hybrid of ionic and covalent character. Breaking these bonds requires a tremendous amount of thermal energy, which is why pure alumina has an extremely high melting point of over 2000°C (3632°F). This is the primary source of its thermal stability.
A Tightly Packed Atomic Lattice
The corundum crystal structure is incredibly dense and tightly packed. This leaves very little empty space within the lattice, making the material physically hard and resistant to deformation. It also makes it difficult for foreign chemicals to penetrate the structure, forming the basis of its chemical resistance.
Why Purity is the Decisive Factor
While the crystal structure provides the theoretical potential for stability, the purity level determines how much of that potential is realized in practice. The difference between a 90% alumina and a 99.6% alumina is the difference between adequacy and extreme performance.
The Role of Impurities as Weak Points
Common impurities in lower-grade alumina include silica (SiO₂) and various alkali metal oxides. These impurities tend to collect at the boundaries between the individual alumina grains.
At high temperatures, these impurities form a glassy, amorphous phase that has a much lower melting point than the pure alumina grains.
How 99.6% Purity Prevents High-Temperature Failure
As temperatures rise, the glassy phase at the grain boundaries softens and eventually melts, acting like a lubricant between the solid alumina grains. This allows the grains to slide past each other, a phenomenon known as creep, causing the entire tube to deform, sag, or fail under load.
By ensuring 99.6% purity, the amount of this glassy phase is minimized. This maintains a strong, interlocking structure between the alumina grains, drastically reducing creep and allowing the tube to retain its structural integrity even in high-vacuum or inert environments approaching its melting point.
Enhancing Chemical Inertness
Impurities are often more chemically reactive than pure alumina. By minimizing these reactive sites, a 99.6% pure tube presents a more uniform and non-reactive surface to its environment. This is critical for preventing corrosion from process chemicals and for maintaining purity in sensitive applications like semiconductor manufacturing.
Understanding the Trade-offs
No material is perfect. Acknowledging the inherent limitations of alumina is crucial for successful implementation.
Brittleness and Mechanical Shock
Like most ceramics, high-purity alumina is extremely hard but also brittle. It has immense compressive strength but will fracture without warning under sharp impacts or significant tensile (pulling) stress. It does not bend or deform before failure.
Thermal Shock Resistance
While alumina has good thermal shock resistance for a ceramic due to its high thermal conductivity and low thermal expansion, it is not immune. Rapid, non-uniform temperature changes can create internal stresses that exceed its strength, causing cracks. Controlled heating and cooling rates are essential.
Specific Chemical Incompatibilities
Despite its excellent general chemical resistance, alumina is not completely inert. It can be attacked by hydrofluoric acid, phosphoric acid, and strong alkaline solutions, especially at elevated temperatures. Certain molten metals, particularly alkali metals, can also be corrosive.
Making the Right Choice for Your Application
Selecting the right material requires matching its properties to the specific stresses of your environment.
- If your primary focus is extreme temperature stability (e.g., furnace processing tubes): The 99.6% purity is non-negotiable, as it directly prevents the high-temperature creep that causes structural failure in lesser-grade ceramics.
- If your primary focus is chemical inertness (e.g., sensor protection, chemical processing): The high purity minimizes potential reaction sites, ensuring both the longevity of the component and the purity of your process.
- If your application involves significant mechanical stress or thermal shock: You must design your system to mitigate these factors, such as by implementing controlled heating cycles and protecting the component from physical impact.
By understanding its atomic-level strengths and practical limitations, you can confidently engineer high-purity alumina into your most demanding systems.
Summary Table:
| Property | Benefit |
|---|---|
| High Purity (99.6%) | Minimizes impurities to prevent high-temperature creep and enhance chemical inertness |
| Strong Ionic-Covalent Bonds | Provides high melting point (>2000°C) and thermal stability |
| Dense Crystal Structure | Offers physical hardness and resistance to deformation and chemical penetration |
| Brittleness | Requires careful handling to avoid mechanical shock and cracking |
| Thermal Shock Resistance | Good for ceramics but needs controlled heating/cooling to prevent stress cracks |
Upgrade your laboratory with KINTEK's advanced high-temperature furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse labs with reliable products like Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures we precisely meet your unique experimental needs, enhancing efficiency and performance in extreme conditions. Contact us today to discuss how our tailored solutions can benefit your specific applications!
Visual Guide
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- How does a tube heating furnace facilitate the carbon coating process? Boost Layered Oxide Conductivity
- What core process conditions does a tube furnace provide? Mastering Catalyst Precursor Treatment
- How is a Vertical Tube Furnace used for fuel dust ignition studies? Model Industrial Combustion with Precision
- What safety and reliability features are incorporated into a vertical tube furnace? Ensuring Safe, Consistent High-Temp Processing
- What function does a tube furnace serve in the PVT growth of J-aggregate molecular crystals? Mastery of Thermal Control



















