At its core, Joule heating is the process by which electrical energy transforms into heat when an electric current passes through a conductor with resistance. In high-temperature heating elements, this fundamental principle is pushed to its extreme, relying on specialized materials that can both generate immense heat and withstand the destructive effects of those temperatures.
The effectiveness of a high-temperature heating element isn't just about its ability to generate heat via resistance. The true challenge lies in selecting materials that remain physically stable and electrically reliable at temperatures exceeding 1000°C.
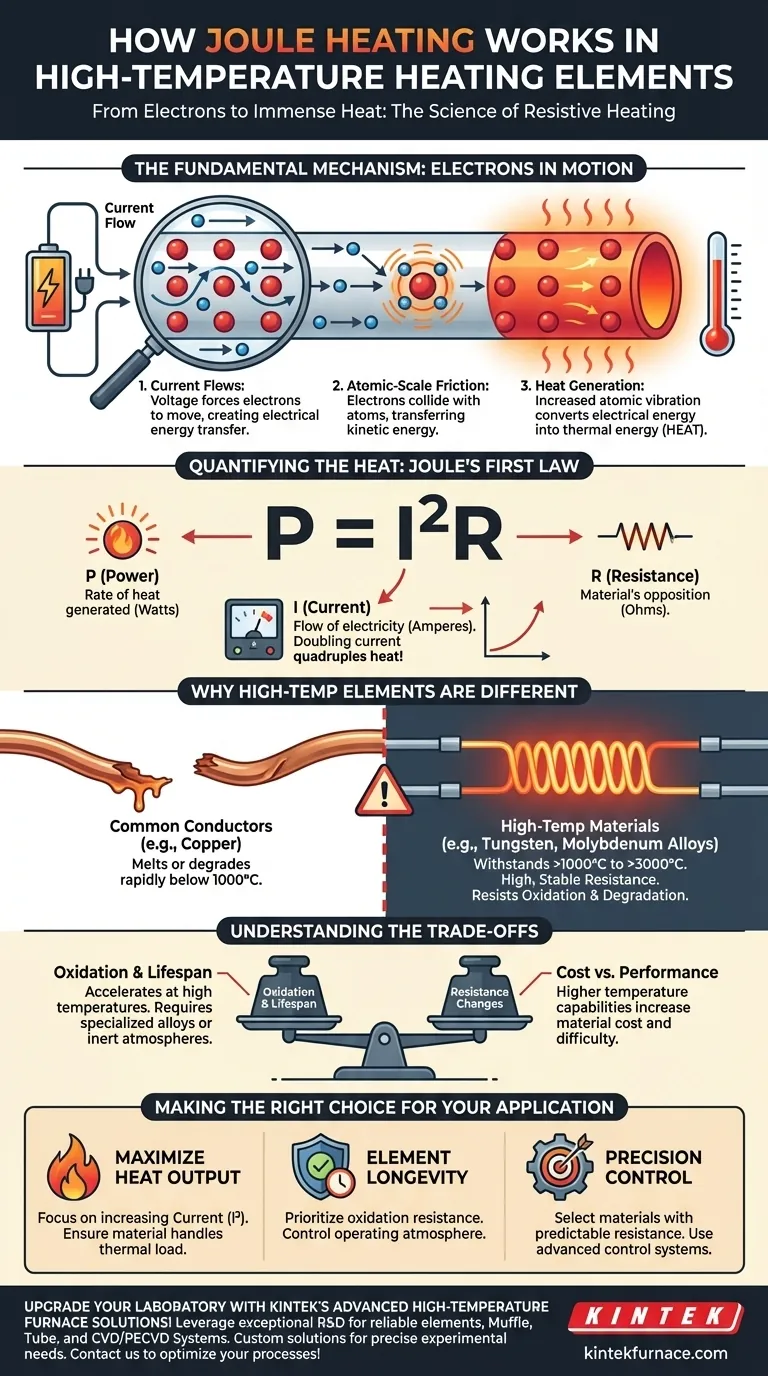
The Fundamental Mechanism: From Electrons to Heat
Joule heating, also known as resistive or ohmic heating, is a predictable and controllable process governed by the laws of physics. It operates on a microscopic level.
The Flow of Current
When voltage is applied across a conductor, it forces electrons to move, creating an electric current. These electrons are the charge carriers responsible for transferring electrical energy through the material.
Atomic-Scale Friction
As these electrons flow, they collide with the atoms and ions that make up the material's crystal lattice structure. Each collision transfers kinetic energy from the electron to the atom, causing the atom to vibrate more intensely.
This widespread, increased atomic vibration is what we perceive and measure as heat. It is a direct conversion of electrical energy into thermal energy.
Quantifying the Heat
This relationship is described by Joule's first law, most commonly expressed as P = I²R.
- P (Power): The rate of heat generated, measured in watts.
- I (Current): The flow of electricity, measured in amperes.
- R (Resistance): The material's opposition to the current, measured in ohms.
This formula reveals that heat output increases exponentially with current. Doubling the current quadruples the heat generated, making current the most significant factor in controlling the element's temperature.
Why High-Temperature Elements Are Different
While a simple wire can demonstrate Joule heating, creating an element for industrial furnaces or reactors requires materials that can perform under extreme conditions.
The Challenge of Extreme Temperatures
Most common conductors, like copper, will melt or rapidly degrade far below the operational range of high-temperature elements, which often start at 1000°C (1832°F) and can exceed 3000°C (5432°F). The primary design challenge is material survival.
The Need for High, Stable Resistance
To generate significant heat efficiently without drawing excessive current, these elements are made from materials with intentionally high electrical resistance. Furthermore, this resistance must remain stable and predictable across a vast temperature range to allow for precise process control.
Material Integrity is Paramount
A successful heating element must not only get hot but also resist melting, sagging, and chemical degradation. Materials are chosen for their high melting points and their ability to resist oxidation or other chemical reactions with the process environment.
Understanding the Trade-offs
Selecting or designing a heating element involves balancing competing factors. Understanding these trade-offs is critical for ensuring reliability and performance.
Oxidation and Lifespan
At high temperatures, the rate of oxidation increases dramatically. This chemical reaction can physically destroy the heating element over time. This is why elements are often made of specialized alloys or used within a vacuum or inert gas atmosphere to prolong their life.
Resistance Changes with Temperature
The resistance of a material is not a constant value; it changes as the material heats up. This "temperature coefficient of resistance" must be accounted for in the power control system to maintain a stable operating temperature.
Cost vs. Performance
There is a direct correlation between an element's maximum operating temperature and its cost. Materials capable of withstanding the most extreme temperatures, like tungsten or molybdenum, are significantly more expensive and difficult to work with than common nickel-chromium alloys.
Making the Right Choice for Your Application
Your final decision must be aligned with your primary technical and operational goals.
- If your primary focus is maximizing heat output: Concentrate on the
P = I²Rrelationship; increasing current is your most powerful lever, provided the element material can handle the thermal load. - If your primary focus is element longevity: Prioritize materials with excellent oxidation resistance for your target temperature range and consider controlling the operating atmosphere.
- If your primary focus is precision temperature control: Select a material with a predictable and well-documented temperature coefficient of resistance and ensure your control system can compensate for it.
By understanding these core principles, you can move from simply using heating elements to intelligently engineering their performance for your specific needs.

Summary Table:
| Aspect | Key Details |
|---|---|
| Mechanism | Electrical current flows through a resistive conductor, causing electron-atom collisions that generate heat via kinetic energy transfer. |
| Governing Law | Joule's first law: P = I²R, where P is power (heat), I is current, R is resistance. |
| Material Challenges | Must withstand >1000°C, resist oxidation, melting, and degradation; requires high, stable resistance. |
| Trade-offs | Balance oxidation resistance, temperature coefficient of resistance, and cost vs. performance for reliability. |
| Application Focus | Maximize heat output (increase current), extend lifespan (use inert atmospheres), or ensure precision control (predictable resistance). |
Upgrade your laboratory with KINTEK's advanced high-temperature furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse labs with reliable heating elements and systems, including Muffle, Tube, Rotary Furnaces, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our deep customization capabilities ensure precise performance for your unique experimental needs, enhancing efficiency and longevity. Contact us today to discuss how we can optimize your heat treatment processes!
Visual Guide
Related Products
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What are the properties and capabilities of Silicon Carbide (SiC) as a heating element? Unlock Extreme Heat and Durability
- Why are SIC heating elements resistant to chemical corrosion? Discover the Self-Protecting Mechanism
- What is the maximum temperature silicon carbide heating elements can withstand? Key Factors for Longevity and Performance
- What are the advantages of using high purity green silicon carbide powder in heating elements? Boost Efficiency and Lifespan
- Why are SiC heating elements considered environmentally friendly? Discover Their Eco-Efficiency & Lifespan Insights



















