A high-alumina crucible is strictly required during the densification of γ-Y1.5Yb0.5Si2O7 ceramics because it provides the necessary thermal stability to withstand sintering temperatures of 1450 °C without deformation. Its primary function is to act as a chemically inert carrier, physically isolating the ceramic "green body" from the furnace heating elements to prevent contamination and ensure the purity of the final product.
The crucible serves as a robust physical and chemical barrier, ensuring that the intense heat required for densification drives grain growth without compromising the sample's structural integrity or chemical composition.

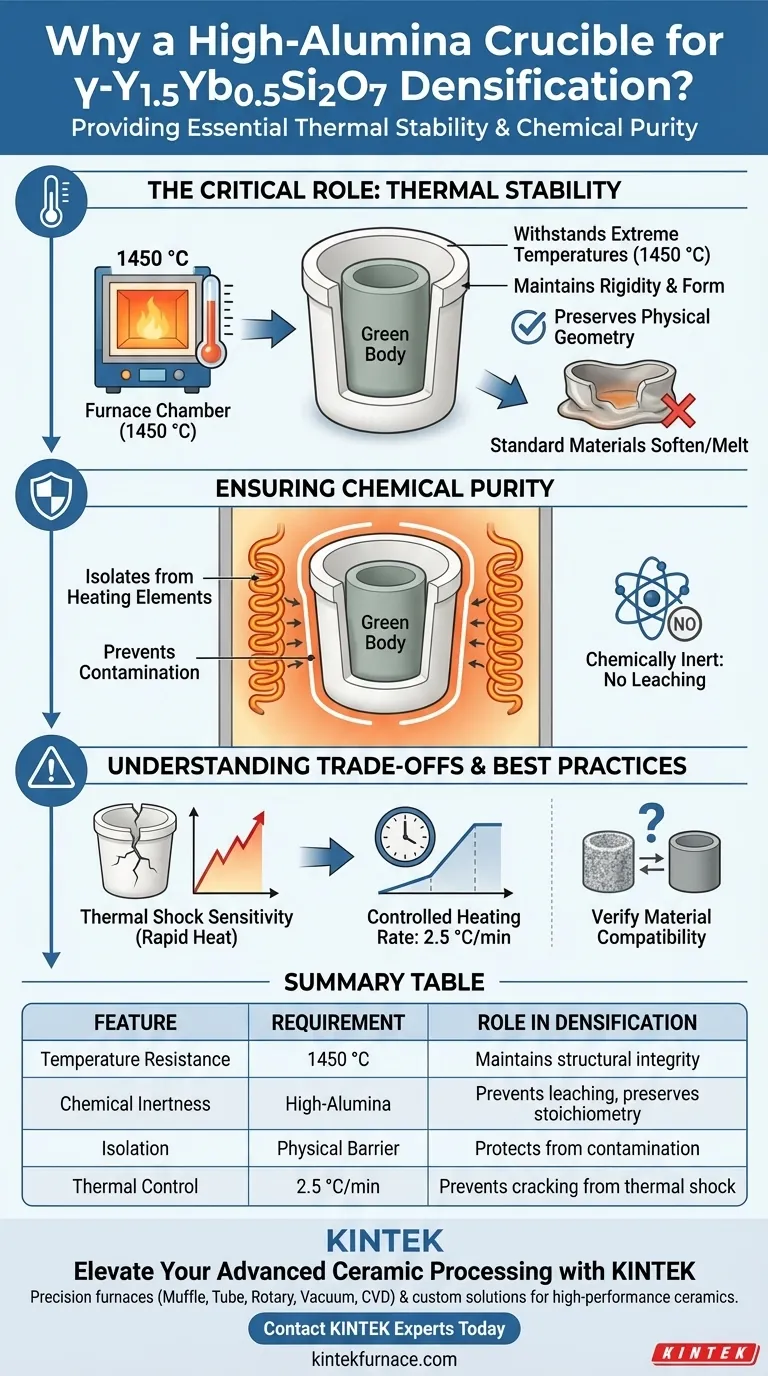
The Critical Role of Thermal Stability
Withstanding Extreme Temperatures
The densification process for γ-Y1.5Yb0.5Si2O7 requires a controlled high-temperature environment reaching 1450 °C.
At this specific temperature, many standard containment materials would soften, warp, or melt. High-alumina crucibles maintain their rigidity and structural form, ensuring the sample remains stable throughout the heating cycle.
Preserving Physical Geometry
The crucible acts as a steadfast carrier for the "green body" (the unfired ceramic powder compact).
By maintaining its shape under heat, the crucible prevents the sample from shifting or deforming due to substrate failure, which is critical for achieving a uniform relative density (such as the 91.2% density noted in successful operations).
Ensuring Chemical Purity
Isolation from Heating Elements
A primary risk during sintering is the interaction between the ceramic sample and the furnace's internal components, specifically the heating elements.
The high-alumina crucible acts as a shield, preventing the γ-Y1.5Yb0.5Si2O7 form making direct contact with the furnace lining or elements. This physical isolation is non-negotiable for high-performance ceramics.
Chemical Inertness
Beyond physical separation, the crucible material itself must be unreactive.
High-alumina is chosen because it is chemically inert regarding this specific ceramic composition. It ensures that no foreign elements leach into the sample, thereby preserving the material's stoichiometry and preventing impurities that could degrade performance.
Understanding the Trade-offs
Thermal Shock Sensitivity
While high-alumina crucibles are heat resistant, they can be sensitive to rapid temperature changes.
It is vital to adhere to precise heating rates—such as 2.5 °C/min—to prevent the crucible itself from cracking. A failure in the crucible wall would immediately expose the sample to the contaminants you are trying to avoid.
Material Compatibility Limits
High-alumina is excellent for γ-Y1.5Yb0.5Si2O7, but it is not a universal solution for all materials.
You must always verify that the specific ceramic powder you are sintering does not react with alumina at elevated temperatures. In this specific case, the reference confirms it is the correct choice, but this compatibility must be re-evaluated if your ceramic composition changes.
Making the Right Choice for Your Project
To ensure the successful densification of your ceramic materials, apply the following guidelines:
- If your primary focus is Chemical Purity: Utilize a high-alumina crucible to create a neutral barrier that prevents reactions with furnace linings and heating elements.
- If your primary focus is Structural Integrity: Ensure your furnace program utilizes a controlled heating rate (e.g., 2.5 °C/min) to protect the crucible from thermal shock while reaching the target 1450 °C.
By selecting the correct containment vessel, you transform a chaotic high-heat environment into a controlled chamber for precise material engineering.
Summary Table:
| Feature | Requirement | Role in Densification |
|---|---|---|
| Temperature Resistance | 1450 °C | Maintains structural integrity without deformation |
| Chemical Inertness | High-Alumina | Prevents leaching and preserves ceramic stoichiometry |
| Isolation | Physical Barrier | Protects sample from heating element contamination |
| Thermal Control | 2.5 °C/min | Prevents crucible cracking due to thermal shock sensitivity |
Elevate Your Advanced Ceramic Processing with KINTEK
Precision densification of high-performance ceramics like γ-Y1.5Yb0.5Si2O7 requires more than just high heat—it demands the right environment. Backed by expert R&D and world-class manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as premium lab high-temp furnaces.
Whether you need custom dimensions or specific atmospheric controls, our systems are fully customizable to meet your unique research and production needs. Ensure chemical purity and structural integrity in every firing cycle.
Ready to optimize your lab's thermal processes?
Contact KINTEK experts today to find the perfect furnace solution for your application.
Visual Guide
References
- Buhao Zhang, Tanvir Hussain. Thermal properties and calcium-magnesium-alumino-silicate (CMAS) interaction of novel γ-phase ytterbium-doped yttrium disilicate (γ-Y1.5Yb0.5Si2O7) environmental barrier coating material. DOI: 10.1007/s42114-024-00879-6
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- Why is a PTFE-lined stainless steel autoclave used for Ni12P5 synthesis? Key Benefits for Nanomaterial Production
- What are tube furnace tubes made of? Select the Right Material for Your Process
- What is the importance of a water-cooled injector in DTF experiments? Ensure Precise Ignition Delay Measurement
- What is the primary function of a radiation pyrometer in validating furnace simulations? Ensure Model Accuracy
- Why is sample handling at high temperatures a risk for the alumina furnace tube? Prevent Thermal Shock Damage
- How does an in-situ reaction chamber in HTXRD facilitate BiFeO3 synthesis study? Mapping Real-Time Phase Evolution
- How does an infrared (IR) pyrometer improve thermal control? Direct Precision for MBE Growth and Annealing
- Why is a vacuum pump used to evacuate the thermal modification chamber? Ensure Safety and Material Integrity



















