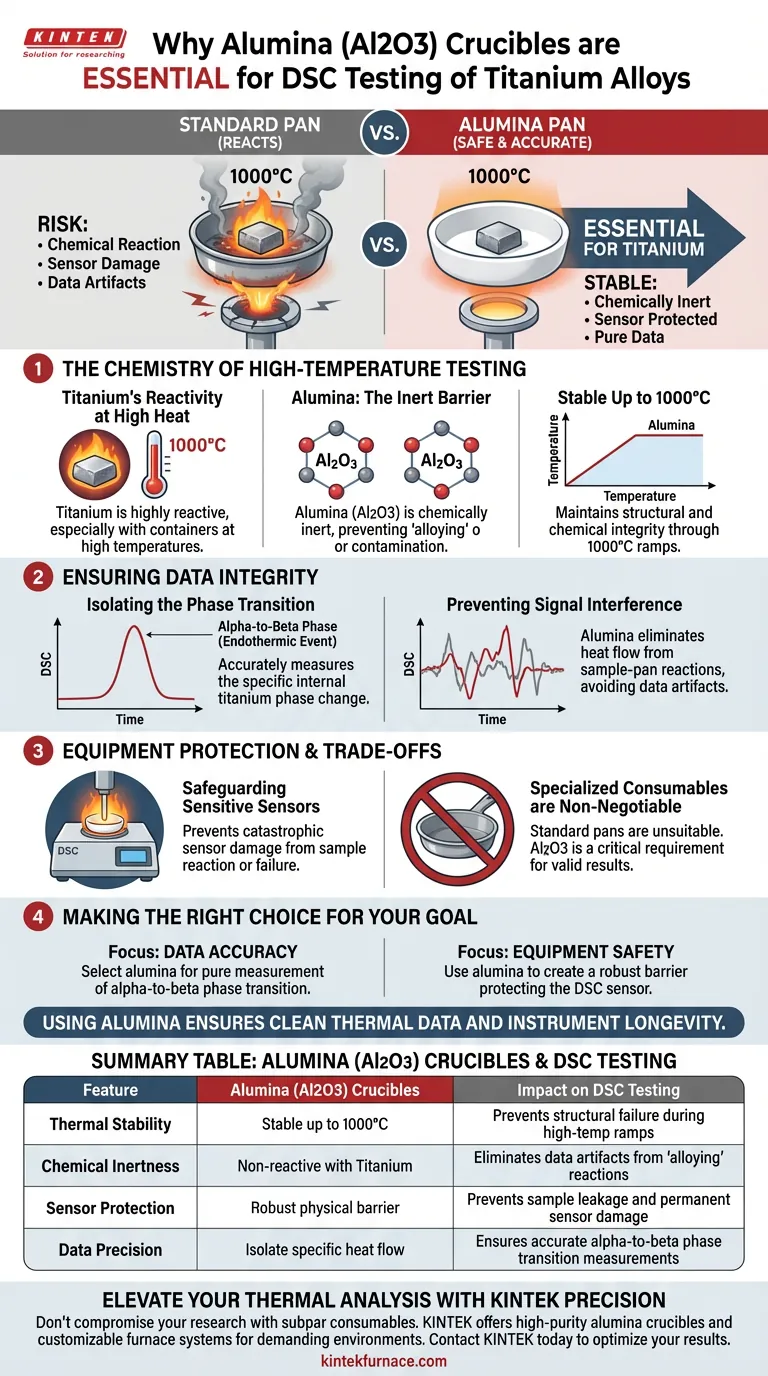
Alumina (Al2O3) sample pans are strictly required for the Differential Scanning Calorimetry (DSC) testing of titanium alloys due to their exceptional thermal stability and chemical inertness. Unlike standard pans, alumina withstands the extreme heat required for titanium testing—up to 1000°C—without chemically reacting with the sample, ensuring the integrity of both your data and your equipment.
The use of alumina ensures that the DSC strictly measures the titanium's internal phase transitions, avoiding the data artifacts and sensor damage that result from chemical reactions between the sample and the crucible.

The Chemistry of High-Temperature Testing
Titanium’s Reactivity at High Heat
Titanium alloys are highly reactive metals, particularly when subjected to elevated temperatures. In a high-heat environment, titanium can easily chemically interact with the container holding it.
The Role of Chemical Inertness
Alumina (aluminum oxide) provides a non-reactive barrier between the sample and the sensor. Because it is chemically inert, Al2O3 prevents the "alloying" or contamination that would occur if the titanium were heated in a less stable vessel.
Stability Up to 1000°C
DSC testing for titanium often requires temperature ramps reaching 1000°C. Alumina remains stable throughout this entire range, maintaining its structural and chemical integrity where other materials might fail or degrade.
Ensuring Data Integrity
Isolating the Phase Transition
The primary goal of DSC in this context is to measure the alpha-to-beta phase transition of the titanium. This is a specific endothermic event that defines the material's properties.
preventing Signal Interference
If the sample pan reacts with the titanium, the DSC will record the heat flow of that reaction. Alumina eliminates this variable, ensuring that the endothermic peaks you see on the graph are exclusively from the titanium's phase change.
Equipment Protection and Trade-offs
Safeguarding Sensitive Sensors
A reaction between a sample and its pan can be catastrophic for the DSC instrument. If the pan fails or the sample reacts through the bottom, it can permanently damage the highly sensitive thermal sensors underneath.
The Necessity of Specialized Consumables
The trade-off in testing reactive alloys is the inability to use standard, general-purpose consumables. You cannot rely on cheaper or more conductive metallic pans; the specific requirement for Al2O3 is a non-negotiable constraint for valid results.
Making the Right Choice for Your Goal
When configuring your DSC experiments for titanium alloys, your pan selection dictates the validity of your results.
- If your primary focus is Data Accuracy: Select alumina pans to ensure the measured heat flow represents only the titanium's alpha-to-beta phase transition, free from reaction artifacts.
- If your primary focus is Equipment Safety: Use alumina crucibles to create a robust barrier that prevents sample leakage and protects the DSC sensor from chemical contamination.
Using the correct crucible material is the single most effective step to ensure clean thermal data and instrument longevity.
Summary Table:
| Feature | Alumina (Al2O3) Crucibles | Impact on DSC Testing |
|---|---|---|
| Thermal Stability | Stable up to 1000°C+ | Prevents structural failure during high-temp ramps |
| Chemical Inertness | Non-reactive with Titanium | Eliminates data artifacts from "alloying" reactions |
| Sensor Protection | Robust physical barrier | Prevents sample leakage and permanent sensor damage |
| Data Precision | Isolate specific heat flow | Ensures accurate alpha-to-beta phase transition measurements |
Elevate Your Thermal Analysis with KINTEK Precision
Don't compromise your sensitive DSC sensors or research integrity with subpar consumables. Backed by expert R&D and manufacturing, KINTEK offers high-purity alumina crucibles, along with customizable Muffle, Tube, Rotary, Vacuum, and CVD furnace systems designed for the most demanding laboratory environments.
Whether you are analyzing reactive titanium alloys or developing next-generation materials, our specialized high-temperature solutions are engineered for accuracy and longevity. Contact KINTEK today to discuss your unique testing needs and discover how our customizable lab equipment can optimize your results.
Visual Guide
References
- Hannah Sims, John J. Lewandowski. The Use of DSC and Independent Oxygen Analyses to Correlate the β Transus Temperature in CP-Ti Grade 2 Materials Processed via Different Techniques. DOI: 10.1007/s11661-025-07922-1
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
People Also Ask
- How are quartz tubes used in laboratory applications? Essential for High-Temp, High-Purity Processes
- How does a heating stage contribute to the quality of multi-material 3D printing? Optimize Precision and Stability
- Why is a laboratory oven utilized during the pre-treatment stage of chicken bone waste pyrolysis?
- What is the primary function of graphitized quartz glass tubes in the synthesis of Bi2Se3-Nd2Se3 alloys?
- Why is a laboratory drying oven or heating plate necessary for Ba7Nb4MoO20? Optimize Slurry Synthesis Results
- What are the critical functions of graphite molds in hot press sintering? Discover their role in densification
- What is the purpose of using a high-purity ceramic crucible with a sealed lid during the thermal treatment of biochar?
- Why is a heating device with magnetic stirring required for Y2O3-MgO precursors? Ensure Perfect Particle Coating


















