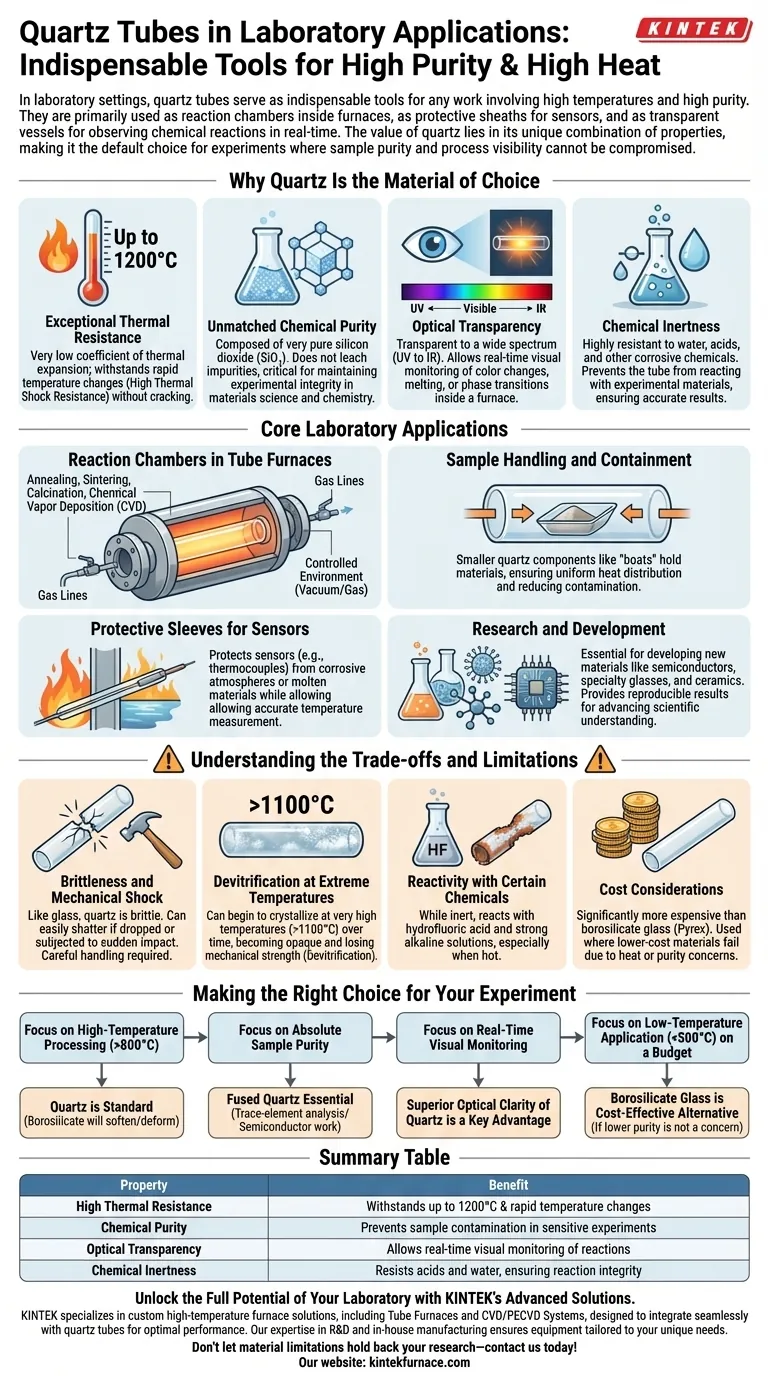
In laboratory settings, quartz tubes serve as indispensable tools for any work involving high temperatures and high purity. They are primarily used as reaction chambers inside furnaces for processes like annealing and sintering, as protective sheaths for sensors, and as transparent vessels for observing chemical reactions in real-time.
The value of quartz in the lab is not due to a single feature, but its unique combination of properties. Its ability to withstand extreme heat, resist chemical attack, and remain transparent makes it the default choice for experiments where sample purity and process visibility cannot be compromised.

Why Quartz Is the Material of Choice
The frequent use of quartz is a direct result of its superior material properties, which are perfectly suited for demanding research environments. Understanding these properties explains why it is chosen over other materials like borosilicate glass.
Exceptional Thermal Resistance
Quartz boasts a very low coefficient of thermal expansion. This means it can endure extremely high temperatures—often up to 1200°C—and withstand rapid temperature changes without cracking. This property is known as high thermal shock resistance.
Unmatched Chemical Purity
Quartz, particularly fused quartz, is composed of very pure silicon dioxide (SiO₂). This high purity ensures that the tube does not leach impurities into a sample during a high-temperature process, which is critical for maintaining experimental integrity in materials science and chemistry.
Optical Transparency
Quartz is transparent to a wide spectrum of light, from ultraviolet (UV) to infrared (IR). This clarity allows researchers to visually monitor processes as they occur inside a furnace, observing color changes, melting, or phase transitions in real time.
Chemical Inertness
Quartz is highly resistant to water, acids, and other corrosive chemicals. This inertness prevents the tube itself from reacting with the experimental materials, ensuring that the results are not skewed by unwanted side reactions.
Core Laboratory Applications
These properties enable quartz tubes to fulfill several critical roles in a modern laboratory, particularly in materials processing and chemistry.
Reaction Chambers in Tube Furnaces
This is the most common application. A quartz tube is placed inside a cylindrical furnace to create a controlled environment for heat treatment. When sealed with flanges, the tube can hold a vacuum or be filled with a specific gas for processes like annealing, sintering, calcination, and chemical vapor deposition (CVD).
Sample Handling and Containment
Smaller quartz components, such as "boats," are used to hold powders or wafers inside a larger tube. This ensures uniform heat distribution around the sample and makes it easy to insert and remove materials without contamination.
Protective Sleeves for Sensors
The material's thermal and chemical resistance makes it an ideal protective sheath. Thermocouples, for example, are often placed inside a thin quartz tube to shield them from corrosive atmospheres or molten materials while still allowing for accurate temperature measurement.
Research and Development
In R&D, quartz tubes are essential for developing new materials like semiconductors, specialty glasses, and ceramics. Their reliability and purity allow for the reproducible results needed to advance scientific understanding.
Understanding the Trade-offs and Limitations
While incredibly useful, quartz is not without its limitations. Acknowledging these trade-offs is key to using it effectively and safely.
Brittleness and Mechanical Shock
Like glass, quartz is brittle. It can easily shatter if dropped or subjected to a sudden mechanical impact. Careful handling is always required.
Devitrification at Extreme Temperatures
When held at very high temperatures (typically above 1100°C) for extended periods, quartz can begin to crystallize in a process called devitrification. This causes it to become opaque and lose its mechanical strength, increasing the risk of failure.
Reactivity with Certain Chemicals
While highly inert, quartz will react with a few substances. It is attacked by hydrofluoric acid and strong alkaline solutions, especially when hot.
Cost Considerations
Quartz is significantly more expensive than standard laboratory glassware like borosilicate (Pyrex). Its use is therefore reserved for applications where lower-cost materials would fail due to high temperatures or concerns about chemical purity.
Making the Right Choice for Your Experiment
Selecting the right material is a critical decision that impacts your budget, safety, and the quality of your data.
- If your primary focus is high-temperature processing (>800°C): Quartz is the standard choice, as common borosilicate glass will soften and deform.
- If your primary focus is absolute sample purity: Fused quartz is essential for trace-element analysis or semiconductor work where even minor contamination is unacceptable.
- If your primary focus is real-time visual monitoring: The superior optical clarity of quartz is a key advantage for observing physical or chemical changes during heating.
- If your primary focus is a low-temperature application on a budget (<500°C): Borosilicate glass is often a more cost-effective alternative, provided its lower chemical purity is not a concern.
By understanding these properties and applications, you can leverage quartz tubes to ensure the accuracy and integrity of your most demanding laboratory work.
Summary Table:
| Property | Benefit |
|---|---|
| High Thermal Resistance | Withstands up to 1200°C and rapid temperature changes |
| Chemical Purity | Prevents sample contamination in sensitive experiments |
| Optical Transparency | Allows real-time visual monitoring of reactions |
| Chemical Inertness | Resists acids and water, ensuring reaction integrity |
Unlock the Full Potential of Your Laboratory with KINTEK's Advanced Solutions
Are you working on high-temperature processes like annealing, sintering, or CVD that demand precision and purity? KINTEK specializes in providing custom high-temperature furnace solutions, including Tube Furnaces and CVD/PECVD Systems, designed to integrate seamlessly with quartz tubes for optimal performance. Our expertise in R&D and in-house manufacturing ensures we can tailor equipment to your unique experimental needs, enhancing accuracy and efficiency.
Don't let material limitations hold back your research—contact us today to discuss how our innovative products can support your laboratory's success!
Visual Guide
Related Products
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What materials are used for the tubes in a High Temperature Tube Furnace? Choose the Right Tube for Your Lab
- What industrial and research applications are tube furnaces used for? Unlock Precise Thermal Processing Solutions
- What is the significance of porcelain furnaces in academic and scientific research? Unlock Innovation with Precise High-Temperature Control
- Why is a high-precision vacuum tube furnace essential for CVD graphene? Master Growth Control & Purity
- What is the function of high-vacuum encapsulated quartz tubes for Ce2(Fe, Co)17? Ensure Phase Purity and Stability



















