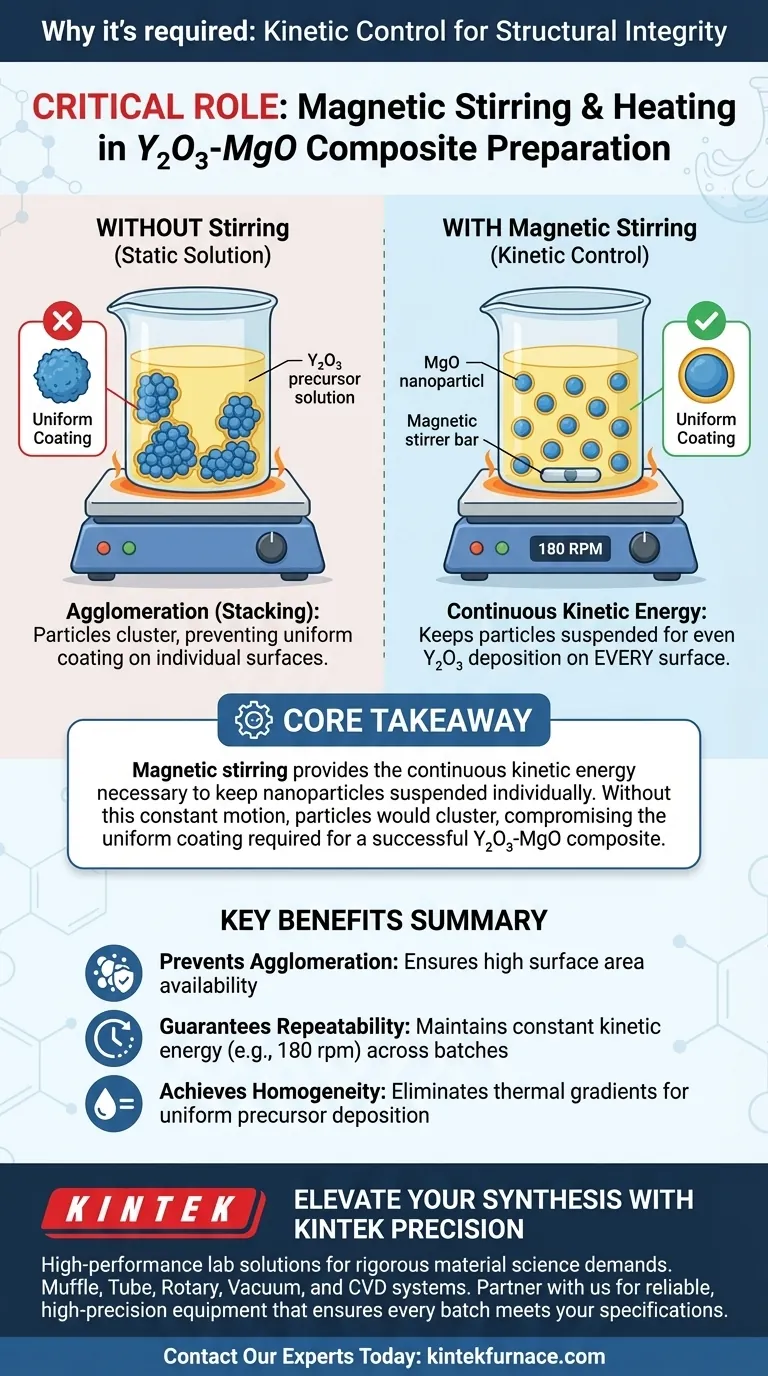
The use of a heating device equipped with magnetic stirring is critical for ensuring the structural integrity of the final composite material. The magnetic stirring function is specifically required to prevent magnesium oxide (MgO) nanoparticles from agglomerating or "stacking" within the solution. By maintaining a constant rotation speed, the device ensures that the yttrium oxide ($Y_2O_3$) precursor deposits evenly onto every surface of the MgO particles.
Core Takeaway: Magnetic stirring provides the continuous kinetic energy necessary to keep nanoparticles suspended individually. Without this constant motion, particles would cluster, preventing the uniform coating required for a successful $Y_2O_3-MgO$ composite.

The Mechanics of Uniform Deposition
To understand why this equipment is required, you must look beyond the chemistry and look at the physical kinetics of the solution.
Combatting Particle Agglomeration
Nanoparticles, such as MgO, have a natural tendency to clump together or "stack" when left static in a mother salt solution.
Magnetic stirring counteracts this by introducing continuous kinetic energy into the suspension. By maintaining a specific rotation speed (e.g., 180 rpm), the stirrer physically forces the particles to remain separate and suspended.
Ensuring Surface Availability
The goal of the synthesis is to create a precursor where $Y_2O_3$ deposits onto the MgO.
If the MgO particles are allowed to agglomerate, the $Y_2O_3$ precursor can only coat the exterior of the clump, leaving the inner particles untouched. Continuous stirring ensures that the entire surface area of every individual MgO particle is exposed to the solution.
Achieving Suspension Homogeneity
Uniformity in the final material starts with uniformity in the liquid phase.
A heating device without stirring would likely result in thermal gradients and particle settling. The magnetic stirrer creates a homogenous environment, ensuring that temperature and chemical concentrations are consistent throughout the entire volume of the liquid.
Understanding the Trade-offs
While magnetic stirring is essential, it introduces variables that must be managed to avoid process failure.
The Risk of Inconsistent Rotation
The effectiveness of this method relies heavily on constant rotation.
If the rotation speed fluctuates or stops, agglomeration can occur almost immediately. Once particles stack, re-suspending them into individual units is difficult, and the uniformity of the subsequent coating will be permanently compromised.
Optimization of Speed
The reference specifically notes a speed of 180 rpm.
Setting the speed too low may fail to generate enough shear force to prevent stacking. Conversely, while not explicitly detailed in the reference, excessive speeds in similar processes can sometimes cause splashing or aeration, suggesting that adhering to a proven parameter like 180 rpm is vital for stability.
Making the Right Choice for Your Synthesis
To apply this to your project, you must view the stirring mechanism not just as a mixer, but as a particle isolator.
- If your primary focus is Coating Uniformity: Ensure your magnetic stirrer is capable of maintaining a constant, uninterrupted RPM throughout the entire heating process.
- If your primary focus is Process Repeatability: Standardize your rotation speed (e.g., at 180 rpm) to ensure that the kinetic energy input remains identical across different batches.
Control the kinetics of your solution, and you control the quality of your composite.
Summary Table:
| Feature | Function in Y2O3-MgO Synthesis | Benefit to Final Composite |
|---|---|---|
| Magnetic Stirring | Prevents MgO nanoparticle stacking/agglomeration | Ensures high surface area availability |
| Constant RPM | Maintains continuous kinetic energy (e.g., 180 rpm) | Guarantees process repeatability |
| Uniform Heating | Eliminates thermal gradients in mother salt solution | Promotes homogenous precursor deposition |
| Kinetic Control | Keeps particles suspended individually | Achieves uniform Y2O3 coating on MgO surfaces |
Elevate Your Composite Synthesis with KINTEK Precision
Achieving uniform nanoparticle coating requires more than just heat—it requires precise kinetic control. KINTEK provides high-performance lab solutions designed to meet the rigorous demands of material science. Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with customizable high-temp furnaces tailored to your unique research needs.
Don't let particle agglomeration compromise your material integrity. Partner with KINTEK for reliable, high-precision equipment that ensures every batch meets your exact specifications.
Contact Our Experts Today to Find Your Custom Solution
Visual Guide
References
- Quanqing Zhang, Nan Wu. Thermal Analysis Kinetics and Luminescence Properties of Y2O3-Coated MgO: Ce+3 Particles. DOI: 10.3390/coatings15020122
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- How does the sealed Alumina Tube structure benefit the design of a reference electrode? Boost Electrolysis Precision
- What is the function of precision magnetic heating stirring equipment in BCZT ceramic preparation? Achieve Homogeneity
- How is the vacuuming operation performed with a water circulating vacuum pump? Master the Liquid Ring Technique
- What is the function of a high-precision constant temperature oven in LIG composite curing? Achieve Perfect Stability
- What role does a rotary evaporator play in microalgae-based nanomaterials? Protect Bio-Reductive Activity for Synthesis
- Why are YSZ milling balls selected for mixing Mn2AlB2 precursor powders? Ensure High-Purity MAB Phase Synthesis
- What are the common types and size ranges of Alumina ceramic tubing? Find the Perfect Fit for Your Lab
- Why are graphite crucible furnaces used in vacuum or protective atmosphere environments? Prevent Oxidation and Ensure Purity



















