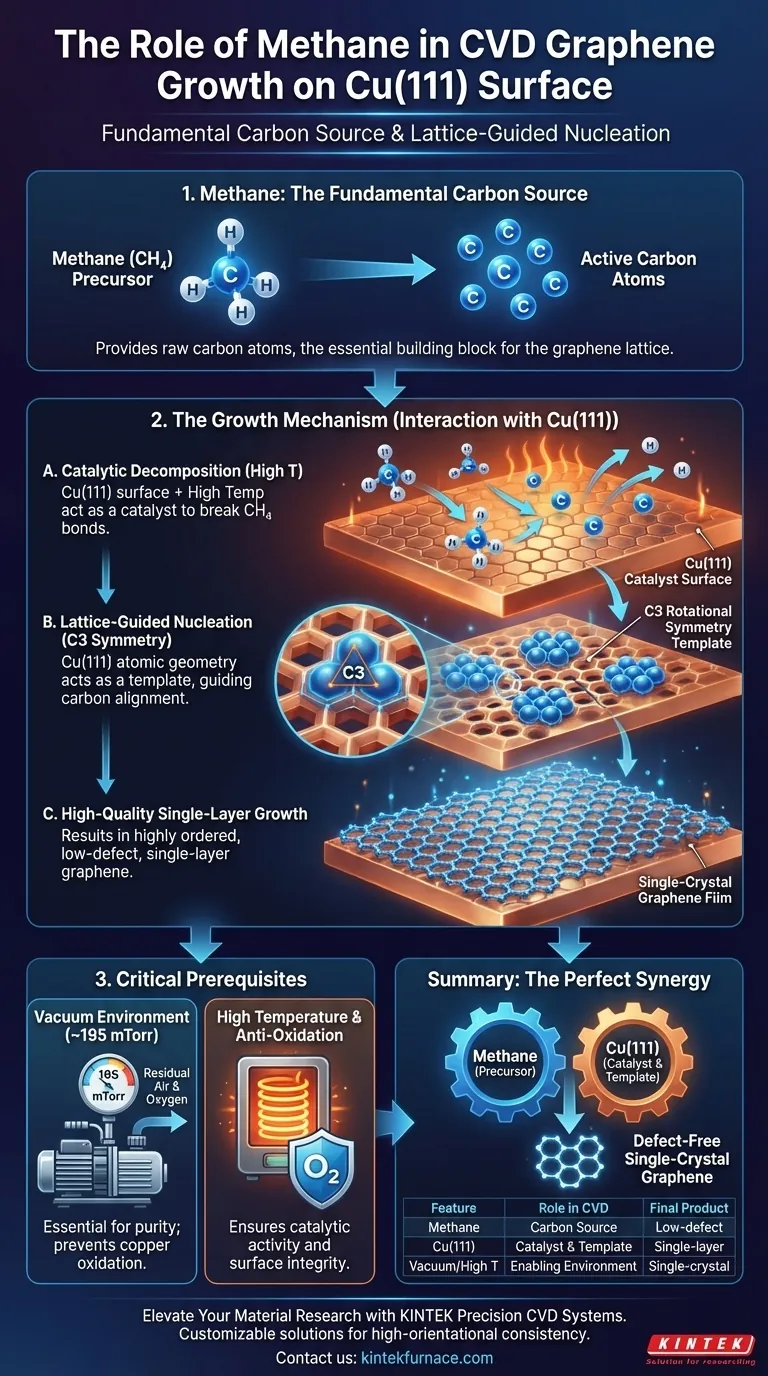
Methane serves as the fundamental carbon source precursor. In a Chemical Vapor Deposition (CVD) system designed for growing graphene on a Cu(111) surface, methane gas ($CH_4$) provides the necessary raw carbon atoms. Without this specific hydrocarbon introduction, there is no material available to construct the graphene lattice.
Core Insight: Methane is not merely a fuel; it is the building block that interacts with the copper catalyst. The Cu(111) surface breaks down the methane and uses its specific atomic geometry to guide the released carbon atoms into a highly ordered, single-layer structure.

The Mechanism of Graphene Growth
To understand why methane is effective, you must look at how it interacts with the substrate at the atomic level.
Catalytic Decomposition
Methane molecules are relatively stable and require energy to break apart.
At high temperatures, the Cu(111) surface acts as a catalyst. It facilitates the decomposition of the methane molecules, stripping away the hydrogen and releasing active carbon atoms onto the surface.
Lattice-Guided Nucleation
Once the carbon atoms are released, they do not settle randomly.
The Cu(111) lattice features C3 rotational symmetry. This specific atomic arrangement acts as a template, forcing the carbon atoms to undergo directional alignment and nucleation.
Achieving High Quality
The interaction between the methane-derived carbon and the Cu(111) template is critical for quality control.
This guided process results in the growth of a single-layer graphene film. Because the carbon atoms align with the underlying copper symmetry, the final film is characterized by low defect density and high orientational consistency.
Prerequisites for Successful Deposition
While methane provides the carbon, the environment must be primed to allow the chemistry to occur.
The Necessity of a Vacuum
Before introducing methane, the system requires a pure growth environment.
An industrial-grade vacuum pump must reduce the base pressure to approximately 195 mTorr. This exhausts residual air that would otherwise interfere with the process.
Preventing Oxidation
The vacuum step is a non-negotiable prerequisite for the heating phase.
Removing air prevents the oxidation of the copper foil. If the copper oxidizes, it cannot effectively catalyze the decomposition of the methane, severely degrading the quality of the resulting graphene.
Making the Right Choice for Your Goal
To optimize your CVD process for single-crystal graphene, consider the following parameters:
- If your primary focus is structural perfection: Prioritize the use of Cu(111) surfaces to fully leverage the C3 symmetry for aligning the carbon atoms released by the methane.
- If your primary focus is process reproducibility: Ensure your system reaches a base pressure of ~195 mTorr to prevent oxidation before the methane is introduced.
The synergy between the methane precursor and the symmetric copper catalyst is the defining factor in producing defect-free single-crystal graphene.
Summary Table:
| Feature | Role in CVD Graphene Growth |
|---|---|
| Methane (CH4) | Primary carbon precursor/building block |
| Cu(111) Surface | Catalyst for decomposition & template for C3 symmetry |
| High Temperature | Provides energy for catalytic decomposition of methane |
| Vacuum Environment | Prevents copper oxidation & ensures high-purity growth |
| Final Product | Low-defect, single-layer single-crystal graphene |
Elevate Your Material Research with KINTEK Precision
Achieving defect-free single-crystal graphene requires more than just the right chemistry—it demands a perfectly controlled thermal environment. KINTEK provides industry-leading high-temperature solutions, including Muffle, Tube, Rotary, Vacuum, and CVD systems, specifically engineered to meet the rigorous demands of graphene synthesis.
Backed by expert R&D and manufacturing, our systems are fully customizable to your unique research or production needs. Ensure your lab has the precision required for high-orientational consistency and low-defect density results.
Ready to optimize your CVD process? Contact us today to discuss your customized furnace solution!
Visual Guide
References
- Jia Tu, Mingdi Yan. Chemical Vapor Deposition of Monolayer Graphene on Centimeter-Sized Cu(111) for Nanoelectronics Applications. DOI: 10.1021/acsanm.5c00588
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Cylindrical Resonator MPCVD Machine System for Lab Diamond Growth
People Also Ask
- What types of materials can be synthesized using CVD? Unlock High-Purity Films for Electronics and More
- What are the benefits of using a CVD furnace? Achieve Atomic-Level Control for Superior Thin Films
- What types of coating precursors are used in the CVD deposition process? Essential Classes for Superior Film Quality
- What roles do quartz boats and quartz tubes play in MoS2 CVD synthesis? Optimize Isotope-Engineered Monolayer Growth
- What are the advantages of TMGa in MOCVD of beta-gallium oxide? Achieve High Growth Rates and Industrial Scalability
- What is Chemical Vapor Deposition (CVD) and what does it produce? Discover High-Purity Thin Films and Coatings
- What types of tools and components are CVD coatings applied to? Boost Durability and Performance in Your Applications
- What are some frequently asked questions about CVD coatings? Discover Key Benefits and Applications



















