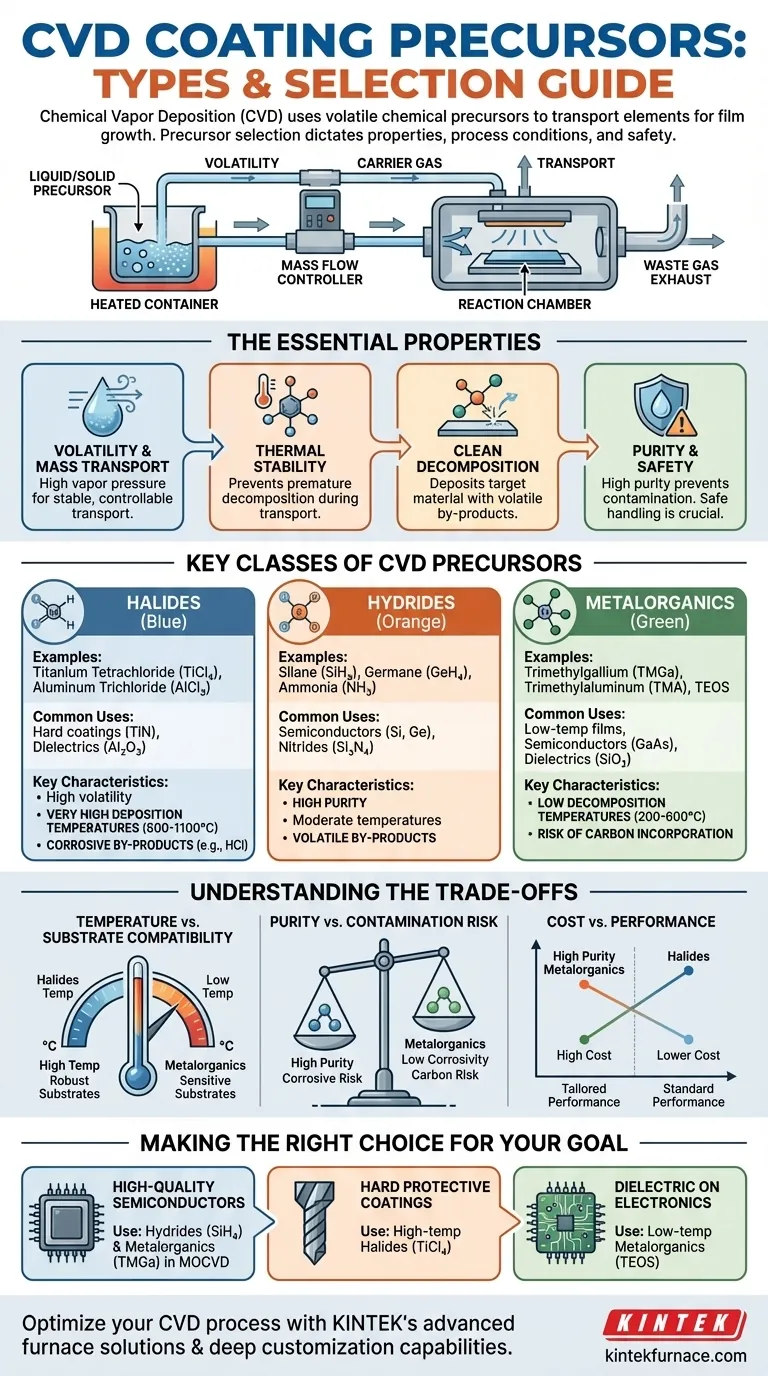
At its core, Chemical Vapor Deposition (CVD) relies on volatile chemical precursors that transport the desired elements to a substrate in gaseous form. The most common classes of precursors are metal halides, hydrides, and metalorganics, each selected based on the required film properties and process conditions.
The selection of a CVD precursor is the single most critical decision in designing a deposition process. It dictates not only the final properties of the coating but also the required temperature, potential contaminants, and safety protocols involved.

The Essential Properties of a CVD Precursor
Before categorizing precursors, it's crucial to understand what makes a compound suitable for CVD. The ideal precursor is a careful balance of several key characteristics.
Volatility and Mass Transport
A precursor must have a sufficiently high vapor pressure at moderate temperatures. This allows it to be easily vaporized and transported into the reaction chamber using a carrier gas, ensuring a stable and controllable flow of material.
Thermal Stability
The compound must be stable enough not to decompose during vaporization or transport. Premature decomposition leads to powder formation in the gas lines instead of film growth on the substrate.
Clean Decomposition
At the substrate surface, the precursor must decompose cleanly and efficiently at a desired temperature. This reaction should deposit the target material while forming volatile by-products that can be easily swept away.
Purity and Safety
Precursors must be available in high purity to prevent unintentional doping or contamination of the final film. Additionally, their handling, toxicity, and corrosive nature are critical safety and equipment considerations.
Key Classes of CVD Precursors
Precursors are generally grouped by their chemical structure. Each class offers a distinct set of advantages and disadvantages.
Halides
This is a classic and widely used precursor category. They are compounds formed between a metal or semi-metal and a halogen element (e.g., Chlorine, Fluorine).
The examples provided in your reference, Titanium Tetrachloride (TiCl₄) for TiN coatings and Aluminum Trichloride (AlCl₃) for Al₂O₃, are perfect illustrations. Halides are often highly volatile but typically require high deposition temperatures.
Hydrides
Hydrides are compounds of an element with hydrogen. They are fundamental for depositing many key semiconductor materials.
Common examples include Silane (SiH₄) for silicon, Germane (GeH₄) for germanium, and Ammonia (NH₃), which serves as a nitrogen source for nitride films like Si₃N₄ or GaN.
Metalorganics
Also known as organometallics, these are compounds with a metal-carbon bond. This is an extremely broad and versatile class, forming the basis of Metalorganic CVD (MOCVD).
They are valued for their lower decomposition temperatures. Key examples include Trimethylgallium (TMGa) for GaAs, Trimethylaluminum (TMA) for Al₂O₃, and Tetraethyl Orthosilicate (TEOS) for silicon dioxide (SiO₂).
Understanding the Trade-offs
No precursor is perfect. The choice always involves balancing competing factors based on the specific application.
Temperature vs. Substrate Compatibility
Halides often produce high-quality, crystalline films but require very high temperatures (600-1100°C). This limits their use to thermally robust substrates like silicon wafers or ceramics.
Metalorganics decompose at much lower temperatures (200-600°C), enabling deposition on temperature-sensitive materials like polymers or pre-processed electronic devices.
Purity vs. Contamination Risk
Halides and hydrides can offer exceptionally high purity. However, halide precursors generate highly corrosive by-products like hydrochloric acid (HCl), which can damage equipment and become incorporated into the film.
Metalorganics avoid corrosive by-products but carry an inherent risk of carbon incorporation into the film, which can degrade electrical or optical properties if not carefully managed.
Cost vs. Performance
High-purity precursors, especially complex metalorganics, can be extremely expensive. For some large-scale industrial applications, a lower-cost precursor may be chosen even if it requires more demanding process conditions or results in slightly lower film quality.
Making the Right Choice for Your Goal
Your choice of precursor is fundamentally tied to the outcome you want to achieve.
- If your primary focus is high-quality epitaxial films for semiconductors: You will likely use a combination of hydrides (SiH₄, AsH₃) and metalorganics (TMGa) in an MOCVD process or halides for certain silicon processes.
- If your primary focus is hard, protective coatings on metal tools: High-temperature CVD using robust and inexpensive halides like TiCl₄ is the industry standard.
- If your primary focus is depositing a dielectric film on a finished electronic device: A low-temperature process using a metalorganic precursor like TEOS is necessary to avoid damaging the underlying circuitry.
Ultimately, the precursor is the foundational ingredient that defines the possibilities and limitations of your entire CVD process.
Summary Table:
| Precursor Class | Key Examples | Common Uses | Key Characteristics |
|---|---|---|---|
| Halides | TiCl₄, AlCl₃ | Hard coatings, TiN, Al₂O₃ | High volatility, high temperature, corrosive by-products |
| Hydrides | SiH₄, NH₃ | Semiconductors, Si, nitrides | High purity, moderate temperature, volatile by-products |
| Metalorganics | TMGa, TEOS | Low-temperature films, GaAs, SiO₂ | Low decomposition temperature, risk of carbon incorporation |
Ready to optimize your CVD process with the right precursors? At KINTEK, we leverage exceptional R&D and in-house manufacturing to provide advanced high-temperature furnace solutions like Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our deep customization capabilities ensure we meet your unique experimental needs, delivering precise temperature control, enhanced film quality, and improved safety. Contact us today to discuss how our tailored solutions can elevate your laboratory's performance and efficiency!
Visual Guide
Related Products
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Inclined Rotary Plasma Enhanced Chemical Deposition PECVD Tube Furnace Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What is the working principle of a CVD tube furnace? Achieve Precise Thin Film Deposition for Your Lab
- What temperature ranges can a CVD Tube Furnace achieve with different tube materials? Unlock High-Temp Precision for Your Lab
- Where is a CVD Tube Furnace commonly used? Essential for High-Tech Materials and Electronics
- What types of atmosphere control does a CVD Tube Furnace support? Master Vacuum and Gas Control for Precision
- Which industries and research fields benefit from CVD tube furnace sintering systems for 2D materials? Unlock Next-Gen Tech Innovations



















