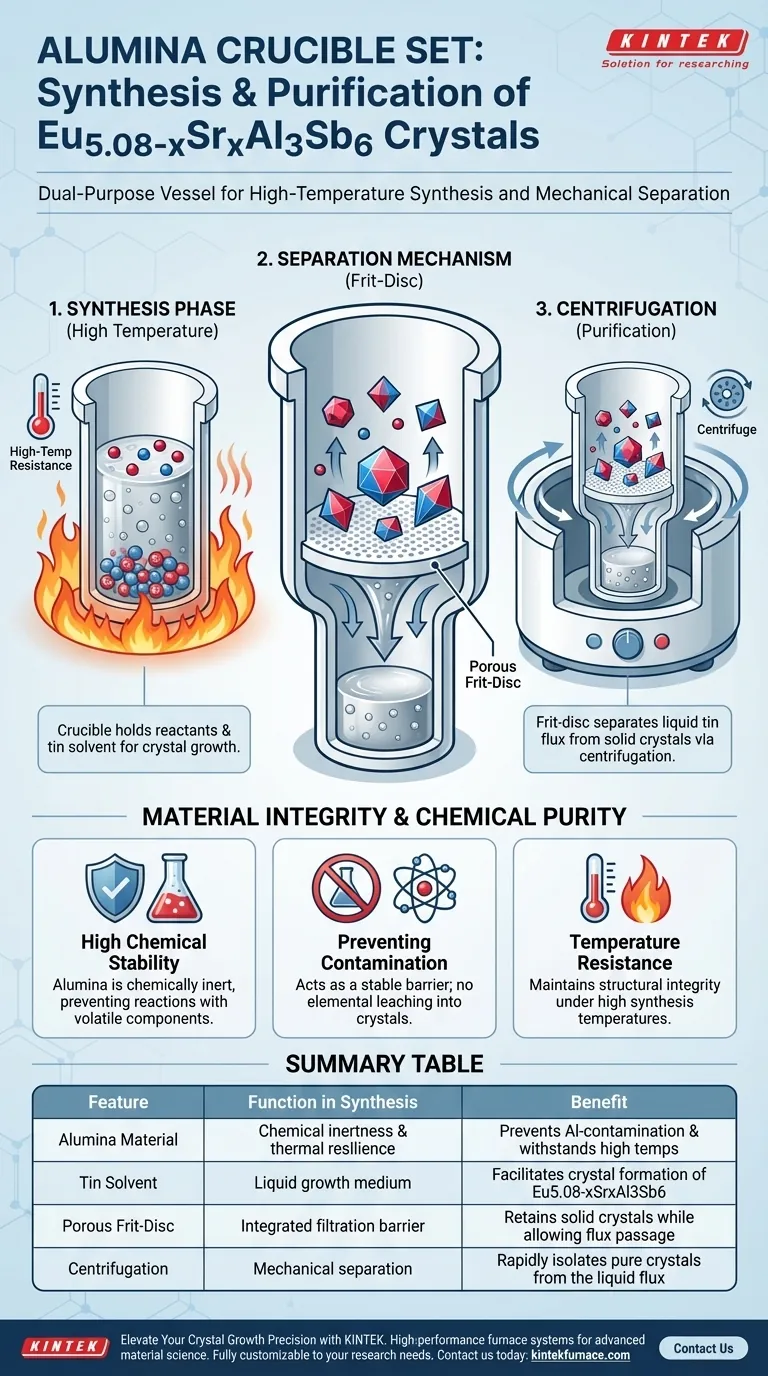
The primary function of the alumina crucible set is to serve as a dual-purpose vessel that manages both the high-temperature synthesis and the subsequent purification of the material. It acts as the core containment unit for the raw reactants and tin solvent during the reaction phase. Crucially, its specialized design allows for the mechanical separation of the final crystals from the liquid flux through centrifugation.
The alumina crucible set solves the challenge of extracting pure crystals from a liquid solvent by integrating a porous filtration system directly into the reaction vessel. This design ensures that the final Eu5.08-xSrxAl3Sb6 product is physically separated from the flux without introducing chemical impurities.

The Mechanics of Separation and Containment
The Role of the Flux Medium
The crucible is designed to hold the raw materials alongside a tin solvent.
This solvent creates the liquid environment necessary for the crystal growth of Eu5.08-xSrxAl3Sb6.
The Integrated Frit-Disc
The defining feature of this crucible set is the inclusion of a frit-disc.
This component provides a porous filtration barrier within the vessel.
Its specific purpose is to facilitate the separation process at the conclusion of the experiment.
Separation via Centrifugation
The synthesis process relies on centrifugation to extract the final product.
During this step, the frit-disc allows the liquid tin flux to pass through its pores while retaining the solid single crystals.
This effectively isolates the desired material from the solvent in a single mechanical step.
Material Integrity and Chemical Purity
High Chemical Stability
The choice of alumina as the construction material is deliberate due to its chemical inertness.
It ensures that the reaction vessel does not react with the volatile components of the synthesis mixture.
Preventing Contamination
A critical requirement for this synthesis is the prevention of aluminum contamination.
The alumina material acts as a stable barrier, ensuring no foreign elements leach into the Eu5.08-xSrxAl3Sb6 crystals.
Temperature Resistance
The vessel is subjected to the high temperatures required to melt the tin solvent and facilitate reaction.
Alumina provides the necessary thermal resilience to maintain structural integrity throughout the heating cycle.
Critical Operational Considerations
Dependence on Frit Integrity
The success of this method relies entirely on the functionality of the porous frit-disc.
If the frit is compromised or the porosity is mismatched to the viscosity of the tin flux, the separation will fail.
The Necessity of Centrifugation
Unlike standard crucible growth, this setup is useless without the accompanying centrifugation step.
The design is engineered specifically for active mechanical separation, rather than passive cooling and decanting.
Applying This Configuration to Your Synthesis
To maximize the quality of your Eu5.08-xSrxAl3Sb6 crystals, consider the following based on your specific objectives:
- If your primary focus is Crystal Purity: Rely on the high chemical stability of the alumina to prevent elemental leaching, ensuring the stoichiometry remains uncompromised by the vessel itself.
- If your primary focus is Efficient Recovery: Utilize the centrifugation capabilities enabled by the frit-disc to cleanly separate the liquid tin flux from the solid crystals immediately after the growth phase.
By leveraging the specific filtration capabilities of this crucible set, you ensure a clean separation of phases while maintaining a chemically inert environment.
Summary Table:
| Feature | Function in Synthesis | Benefit |
|---|---|---|
| Alumina Material | Chemical inertness & thermal resilience | Prevents Al-contamination & withstands high temps |
| Tin Solvent | Liquid growth medium | Facilitates crystal formation of Eu5.08-xSrxAl3Sb6 |
| Porous Frit-Disc | Integrated filtration barrier | Retains solid crystals while allowing flux passage |
| Centrifugation | Mechanical separation | Rapidly isolates pure crystals from the liquid flux |
Elevate Your Crystal Growth Precision with KINTEK
High-performance synthesis requires more than just raw materials—it demands the right thermal environment. Backed by expert R&D and world-class manufacturing, KINTEK provides high-quality Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the rigorous demands of advanced material science.
Whether you are synthesizing complex crystals like Eu5.08-xSrxAl3Sb6 or require a specialized high-temperature lab furnace, our systems are fully customizable to your unique research needs. Contact us today to discover how KINTEK's heating solutions can enhance your laboratory's efficiency and ensure the chemical integrity of your results.
Visual Guide
References
- Luis Garay, Susan M. Kauzlarich. Interplay of Crystal Structure and Magnetic Properties of the Eu<sub>5.08-x</sub>Sr<sub><i>x</i></sub>Al<sub>3</sub>Sb<sub>6</sub> Solid Solution. DOI: 10.1021/acs.inorgchem.4c04927
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- Why are high-purity graphite crucibles with lids used for ilmenite reduction? Control Your Micro-Reducing Atmosphere
- What are the typical size ranges available for quartz tubes used in laboratory furnaces? Find Your Perfect Fit for High-Temp Applications
- What role does a high-purity graphite mold play during the SPS of TiB2-SiC? Expert Material Densification Insights
- What are the advantages of a water circulating vacuum pump? Superior for Wet, Corrosive Gas Handling
- What functions do carbon black and carbon fiber felt serve as insulation? Maximize Efficiency in 3000°C Furnaces
- Why are high-purity alumina tubes and crucibles preferred for high-temperature smelting? Ensure Maximum Sample Purity
- What role does an alumina crucible play during the gas nitriding process for stainless steel? Ensure Surface Purity
- What are some specialized applications of quartz tubes? Essential for High-Temperature and High-Purity Processes



















