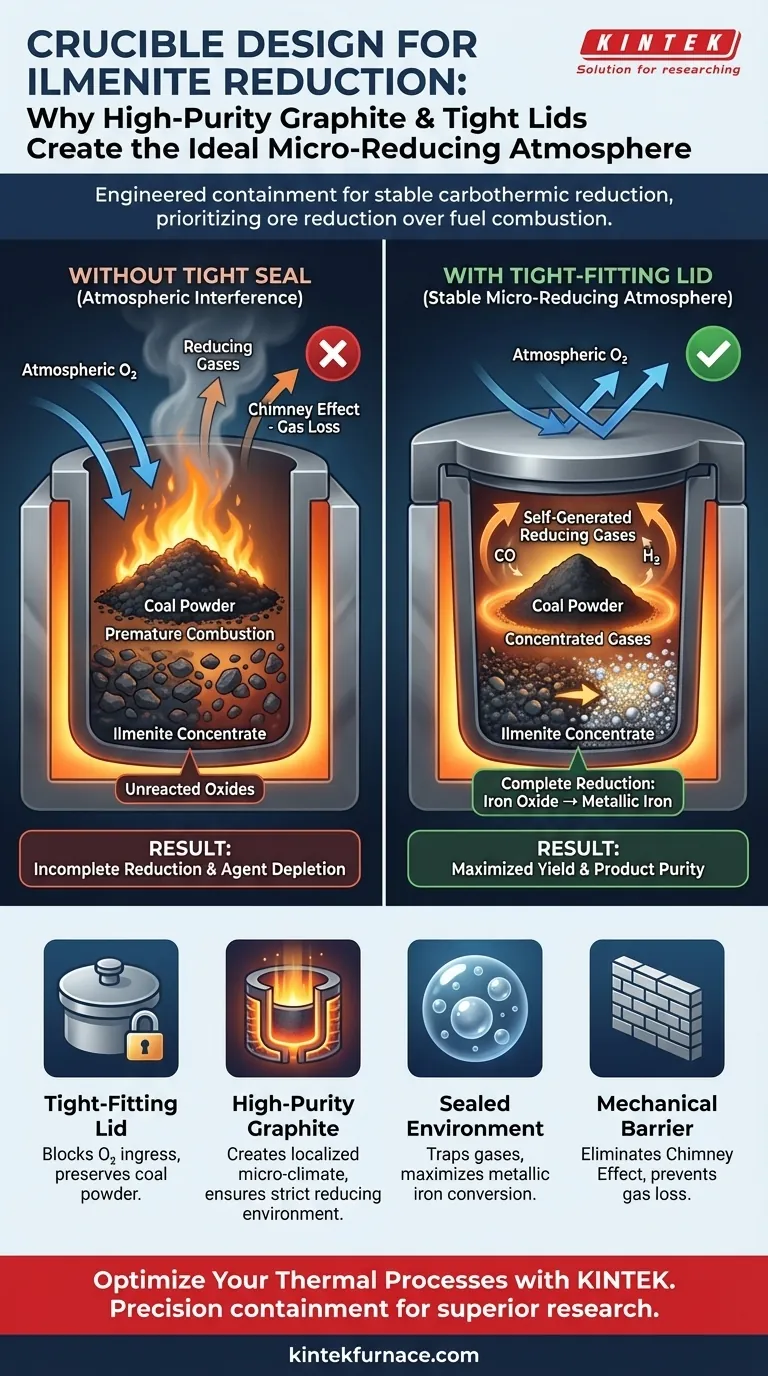
High-purity graphite crucibles paired with tight-fitting lids are utilized primarily to engineer a stable micro-reducing atmosphere. This physical barrier restricts the ingress of atmospheric oxygen, ensuring that the coal powder—acting as the reducing agent—is preserved for the reaction rather than burning away prematurely. Furthermore, this sealed environment captures self-generated reducing gases, maintaining the high concentrations required to thoroughly reduce iron oxides within the ilmenite to a metallic state.
The integrity of the reaction vessel dictates the efficiency of the chemical conversion. A tight graphite seal effectively isolates the system, prioritizing the reduction of ore over the combustion of fuel.

Creating the Necessary Chemical Environment
To understand why this specific hardware is used, you must look at the delicate balance required in carbothermic reduction. The goal is to remove oxygen from the ore, not to burn the fuel source with atmospheric air.
Preventing Reducing Agent Depletion
The primary threat to the reduction process is the uncontrolled entry of external air. Without a physical barrier, oxygen infiltrates the chamber and reacts directly with the coal powder. This oxidation depletes the reducing agent before it can interact with the ilmenite, causing the reaction to stall or fail completely.
Concentrating Reducing Gases
The reduction of ilmenite relies heavily on gases generated inside the crucible during heating. The tight-fitting lid ensures these reducing gases remain trapped within the reaction zone rather than dissipating into the furnace. maintaining a high concentration of these gases is critical for driving the complete transformation of iron oxides into metallic iron.
Establishing the Micro-Reducing Atmosphere
The combination of the graphite material and the seal creates a localized "micro-climate" inside the crucible. This allows the internal chemistry to differ significantly from the general atmosphere of the furnace. It ensures the environment remains strictly reducing, regardless of external conditions.
Understanding the Trade-offs
While using high-purity graphite with tight lids is the standard for quality, there are operational considerations to keep in mind.
The Risk of Pressure Buildup
A seal that is too perfect can theoretically lead to pressure issues if gas generation is rapid. However, standard graphite lids generally allow just enough permeability or slight venting to prevent rupture while blocking bulk air entry.
The Consequence of Loose Fittings
Conversely, a lid that does not fit tightly renders the graphite crucible nearly useless for this specific application. A loose fit permits the "chimney effect," drawing out valid reducing gases and pulling in destructive oxygen. This results in incomplete reduction, leaving unreacted oxides in the final product.
Making the Right Choice for Your Process
The selection of crucible assembly is not just about holding material; it is about controlling chemistry.
- If your primary focus is maximizing yield: Ensure the lid tolerance is tight to prevent the loss of the coal powder reducing agent.
- If your primary focus is product purity: Rely on the containment provided by the lid to ensure the iron oxides are thoroughly reduced to a metallic state without atmospheric interference.
The mechanical design of your crucible is the first line of defense in chemical process control.
Summary Table:
| Feature | Function in Carbothermic Reduction | Benefit |
|---|---|---|
| Tight-Fitting Lid | Blocks atmospheric oxygen ingress | Prevents premature burning of coal powder |
| High-Purity Graphite | Creates a localized micro-climate | Ensures environment remains strictly reducing |
| Sealed Environment | Traps self-generated reducing gases | Maximizes iron oxide to metallic iron conversion |
| Mechanical Barrier | Eliminates the "Chimney Effect" | Prevents loss of vital gases and incomplete reduction |
Optimize Your Thermal Processes with KINTEK
Precision in chemical reduction begins with superior containment. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, along with customizable lab high-temp furnaces tailored to your unique research needs. Whether you are refining ilmenite or developing advanced materials, our hardware ensures the stable environment your experiments demand.
Ready to elevate your lab's efficiency? Contact our technical experts today to find the perfect customizable solution for your process.
Visual Guide
References
- Efficiency of Soda-Technology Carbothermal Smelting of Thermoactivated Ilmenite Concentrate with Aluminosilicate Mineralization. DOI: 10.3390/min15090906
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Magnesium Extraction and Purification Condensing Tube Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What is the main purpose of BN coating on graphite in Ti-6Al-4V hot pressing? Ensure Purity & Easy Release
- What are the key properties of alumina ceramic tubes? Unlock High-Temp Performance for Your Lab
- What are the functions of a high-purity graphite mold during the SPS process? Beyond Containing the Powder
- What are the thermal properties of alumina tubes? Discover Their High-Temp Durability and Stability
- What are the advantages of nickel crucibles for KOH activation? Ensure High Purity & Thermal Stability up to 700°C
- How are quartz tubes used in laboratory applications? Essential for High-Temp, High-Purity Processes
- Why is a mass flow controller essential in the tracer method? Precision Data for Pyrolysis Gas Flow
- What is the function of a high-pressure stainless steel autoclave? Master Hydrothermal Synthesis of Nanomaterials



















