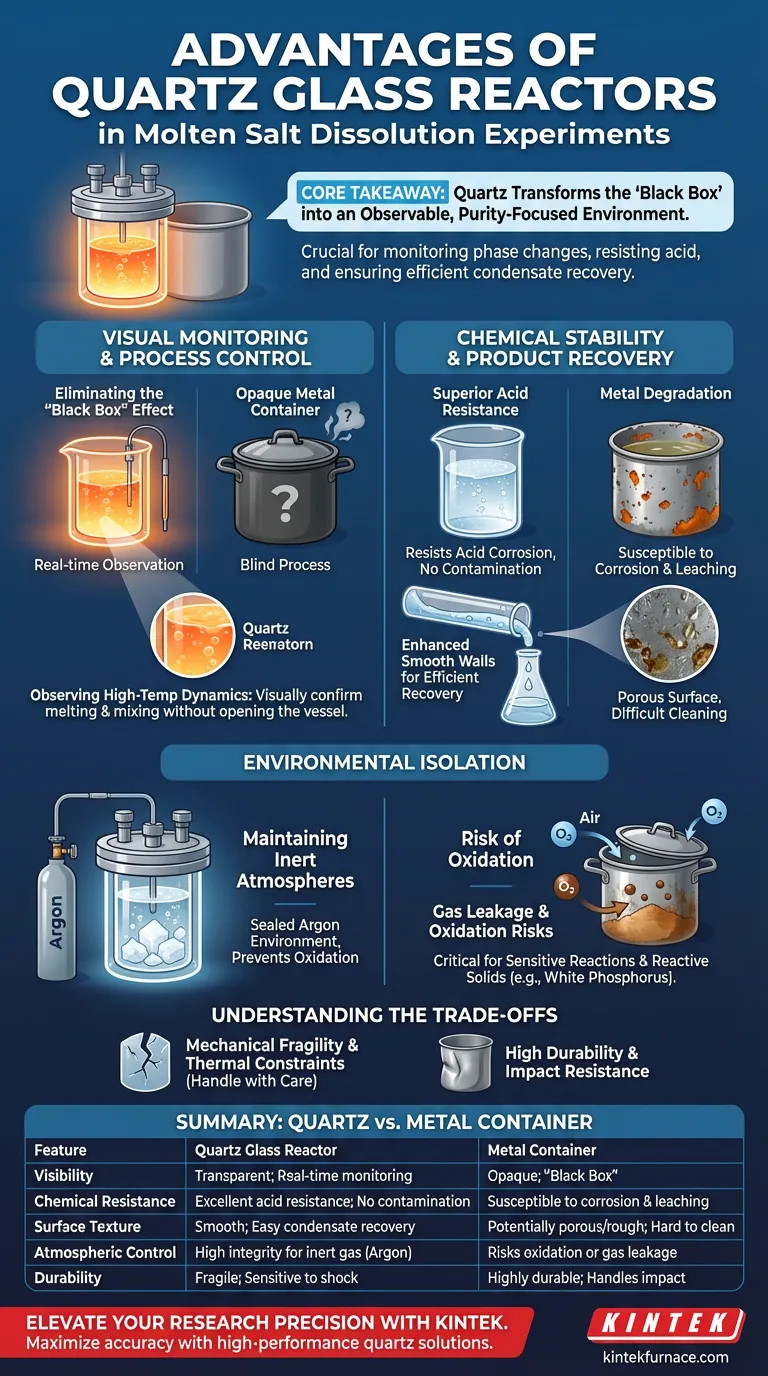
The primary advantages of using a quartz glass reactor over a metal container in molten salt dissolution experiments are its optical transparency and superior chemical inertness. Quartz allows for the direct visual monitoring of high-temperature melting processes while simultaneously resisting acid corrosion and maintaining the integrity of inert atmospheres.
Core Takeaway Quartz transforms the reactor from a "black box" into an observable environment, crucial for monitoring phase changes and reaction behaviors. Its smooth, acid-resistant surface is specifically advantageous for ensuring the purity of the reaction and the efficient recovery of volatile condensates.

Visual Monitoring and Process Control
Eliminating the "Black Box" Effect
The most immediate benefit of quartz is transparency. Unlike metal containers, quartz allows you to directly observe the physical state of the molten salt and the dissolution process in real-time.
Observing High-Temperature Dynamics
This visibility is critical when working with high-temperature melting processes. It allows you to visually confirm when melting is complete and monitor mixing dynamics without opening the vessel or relying solely on thermal sensors.
Chemical Stability and Product Recovery
Superior Acid Resistance
Quartz offers excellent resistance to acid corrosion. In experiments involving acidic environments or byproducts, metal containers can degrade, potentially contaminating the sample or failing structurally.
Enhanced Volatile Collection
The smooth internal walls of a quartz reactor provide a significant advantage for post-reaction analysis. Volatile products that condense on the vessel walls are easier to collect and analyze compared to the potentially rougher or porous surfaces of metal containers.
Environmental Isolation
Maintaining Inert Atmospheres
Quartz is highly effective at sealing and maintaining a specific gas environment, such as an argon atmosphere, even at high temperatures. This isolation is critical for sensitive reactions that cannot be exposed to air.
Preventing Oxidation
In the specific context of producing reactive substances like white phosphorus, this atmospheric control is non-negotiable. The quartz barrier effectively isolates the reaction, preventing the oxidation of the final product that would occur if air leaked into the system.
Understanding the Trade-offs
Mechanical Fragility
While quartz offers superior chemical and optical properties, it lacks the mechanical durability of metal. It requires careful handling to avoid breakage, particularly during setup and cleaning.
Thermal Constraints
Although quartz handles high temperatures well, it does not possess the ductility of metal. Operators must be mindful of thermal shock or physical impact that a metal canister would otherwise absorb without failure.
Making the Right Choice for Your Goal
When designing your molten salt experiment, the choice of reactor material defines your capabilities.
- If your primary focus is process visibility: Choose quartz to enable direct observation of phase changes and mixing dynamics.
- If your primary focus is product purity and recovery: Choose quartz to utilize its acid resistance and smooth walls for easy collection of volatile condensates.
- If your primary focus is handling reactive solids (like White Phosphorus): Choose quartz to ensure a strictly maintained inert argon atmosphere that prevents oxidation.
The shift from metal to quartz is a shift from durability to precision, visibility, and chemical purity.
Summary Table:
| Feature | Quartz Glass Reactor | Metal Container |
|---|---|---|
| Visibility | Transparent; allows real-time monitoring | Opaque; "Black Box" environment |
| Chemical Resistance | Excellent acid resistance; no contamination | Susceptible to corrosion and leaching |
| Surface Texture | Smooth; easy recovery of condensates | Potentially porous/rough; harder to clean |
| Atmospheric Control | High integrity for inert gas (Argon) sealing | Risks oxidation or gas leakage at joints |
| Durability | Fragile; sensitive to thermal/physical shock | Highly durable; handles physical impact |
Elevate Your Research Precision with KINTEK
Maximize your experimental accuracy with high-performance quartz solutions tailored for molten salt dissolution. Backed by expert R&D and manufacturing, KINTEK offers a wide range of customizable Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the rigorous demands of modern laboratories. Whether you need the optical clarity of quartz or the heavy-duty performance of specialized high-temp furnaces, our team provides the engineering expertise to support your unique needs.
Ready to transform your process from a black box to a transparent success? Contact us today to discuss your custom furnace requirements!
Visual Guide
References
- Yuxiang Zhong, Xiao Yang. Extracting White Phosphorus from AlPO<sub>4</sub> through Molten Salt Processing. DOI: 10.5796/electrochemistry.24-69001
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- Why is a high vacuum pumping system necessary for carbon nanotube peapods? Achieve Precise Molecular Encapsulation
- What is the role of a mechanical vacuum pump in the preparation of FeAl alloys? Achieve 10⁻² Pa for Pure Synthesis
- Why are a press and pelletizing molds necessary when preparing pellets for magnesium smelting? Ensure Smelting Efficiency and Control
- What is the purpose of using a corundum crucible and graphite powder? Optimize Your High-Entropy Alloy Annealing
- Why are high-purity quartz tubes and quartz boats preferred for plastic pyrolysis? Ensure Precise, Pure Results
- What role does a copper mold play in the formation of glass samples? Master Rapid Quenching & Amorphous Solidification
- Why are desiccators containing saturated salt solutions used when evaluating the hygroscopicity of modified wood?
- What are the advantages of 0.7 mm quartz capillaries for SXRD? Optimize High-Energy In-Situ X-ray Experiments



















