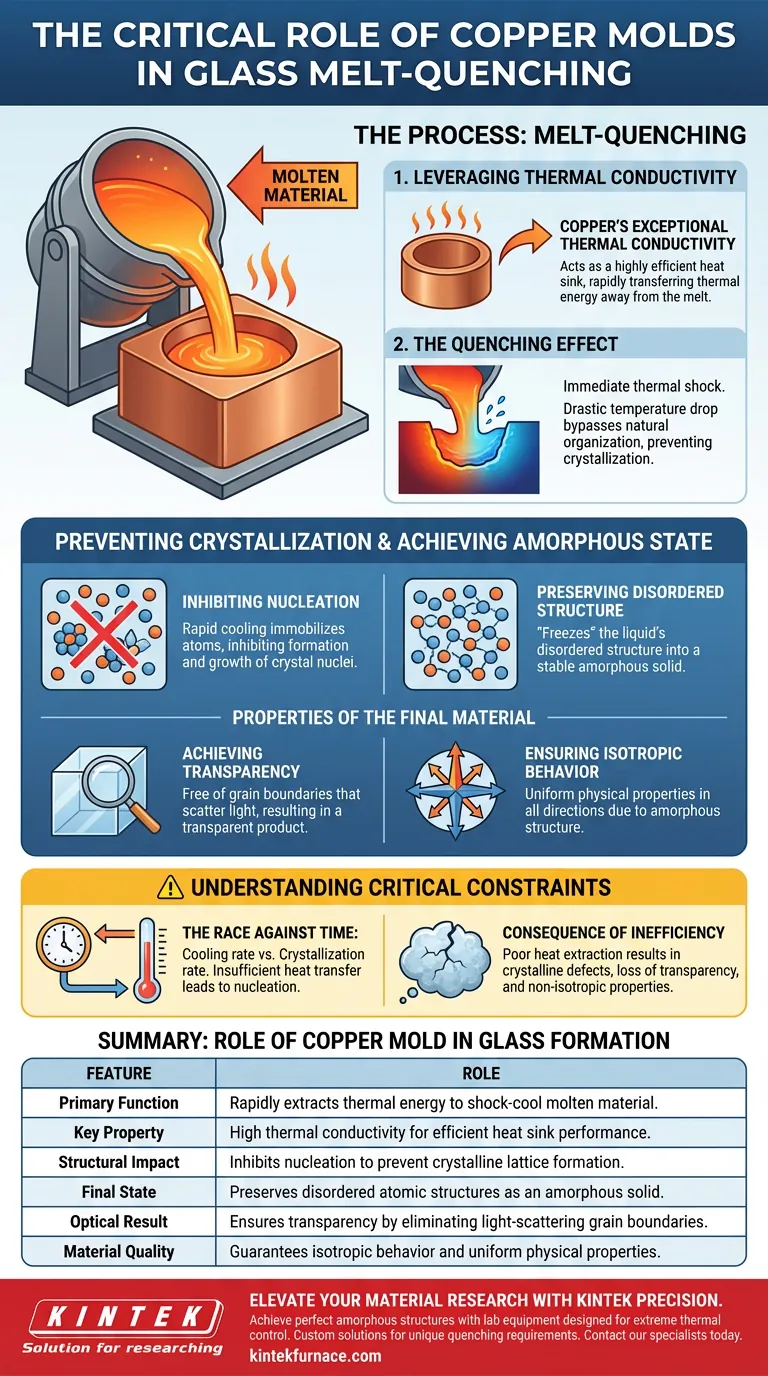
In the melt-quenching process, a copper mold serves as a critical heat dissipation interface designed to rapidly extract thermal energy. Its primary role is to leverage copper's high thermal conductivity to shock-cool molten material, preventing the natural formation of crystalline structures during solidification.
By enabling extremely high cooling rates, the copper mold prevents the ordered arrangement of atoms. This inhibits crystal nucleation, freezing the molten liquid’s disordered structure into a stable, transparent, and amorphous glass solid.
The Mechanics of Rapid Cooling
Leveraging Thermal Conductivity
The effectiveness of the melt-quenching method relies entirely on how fast heat can be removed from the molten glass. Copper is utilized specifically for its exceptional thermal conductivity.
This property allows the mold to act as a highly efficient heat sink. It transfers thermal energy away from the melt much faster than other mold materials would allow.
The Quenching Effect
As the molten material touches the copper surface, it experiences a drastic drop in temperature. This is not a gradual cooling process; it is an immediate thermal shock.
This rapid cooling is necessary to bypass the material's natural tendency to organize itself as it solidifies.
Preventing Crystallization
Inhibiting Nucleation
In a slow-cooling environment, atoms have time to arrange themselves into ordered, crystalline patterns. The copper mold disrupts this by inhibiting the formation and growth of crystal nuclei.
By removing heat instantly, the atoms are immobilized before they can migrate into a lattice structure.
Preserving Disordered Structure
The ultimate goal of using the copper mold is to "freeze" the liquid state of the material.
The mold preserves the disordered structure characteristic of the melt. Instead of becoming a crystal, the material solidifies as an amorphous solid.
Properties of the Final Material
Achieving Transparency
Because the copper mold prevents crystallization, the resulting solid is free of grain boundaries that typically scatter light.
This results in a transparent final product, which is a hallmark of high-quality glass formation.
Ensuring Isotropic Behavior
The rapid cooling ensures the glass is isotropic. This means the material possesses uniform physical properties in all directions.
This uniformity is a direct result of the amorphous, non-crystalline structure maintained by the mold's cooling efficiency.
Understanding the Critical Constraints
The Race Against Time
The process is effectively a race between the cooling rate and the rate of crystallization. If the heat transfer is insufficient, the material will begin to nucleate.
The Consequence of Inefficiency
If the interface between the melt and the copper is poor, or if the heat extraction is too slow, the "amorphous" goal fails. The material will develop crystalline defects, losing its transparency and isotropic nature.
Making the Right Choice for Your Goal
To ensure you achieve the desired material properties, consider how the cooling rate impacts your specific objectives:
- If your primary focus is optical clarity: Ensure the mold surface provides maximum contact to prevent crystal growth that causes opacity.
- If your primary focus is structural uniformity: Rely on the high thermal conductivity of copper to guarantee the material remains isotropic and amorphous throughout.
The copper mold is not just a container; it is the active tool that forces the material to remain amorphous by denying it the time to crystallize.
Summary Table:
| Feature | Role of Copper Mold in Glass Formation |
|---|---|
| Primary Function | Rapidly extracts thermal energy to shock-cool molten material. |
| Key Property | High thermal conductivity for efficient heat sink performance. |
| Structural Impact | Inhibits nucleation to prevent crystalline lattice formation. |
| Final State | Preserves disordered atomic structures as an amorphous solid. |
| Optical Result | Ensures transparency by eliminating light-scattering grain boundaries. |
| Material Quality | Guarantees isotropic behavior and uniform physical properties. |
Elevate Your Material Research with KINTEK Precision
Achieve perfect amorphous structures with lab equipment designed for extreme thermal control. Backed by expert R&D and world-class manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique quenching and synthesis requirements.
Ready to optimize your glass formation process? Contact our specialists today to discover how our high-temperature furnaces and custom solutions can enhance your laboratory's efficiency and output.
Visual Guide
References
- Fathy Abdel-Wahab, Heba Abdelmaksoud. Investigation of oxygen defects in chromium-doped borosilicate glass co-doped with alkali metal (Na2O) and transition metal (ZnO) for photonic applications. DOI: 10.1007/s00339-024-08114-1
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Induction Melting Furnace
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
People Also Ask
- What role does the planetary ball mill play in LLZO mixing? Unlock High-Performance Solid-State Electrolyte Synthesis
- Why is the selection of crucibles with specific internal linings necessary? Protect Purity in Superalloy Melting
- Why is temperature-controlled heating equipment required for calcium perrhenate? Ensure Rhenium Stability at 140 °C
- What is the role of a laboratory oven in the pre-treatment of Date Palm Stones? Enhance Torrefaction & Grinding Efficiency
- Why use graphite crucibles for sludge ash reduction? Unlock Superior Reduction & Heat Resistance
- Why are alumina crucibles used for CoNb2O6 synthesis? Ensure High-Purity Ceramic Powder Production
- How does a lab vacuum pump work? Understanding the Liquid Piston Mechanism
- How do alumina ceramic furnace tubes compare to other materials like quartz or silicon carbide? Choose the Best for Your High-Temp Needs

