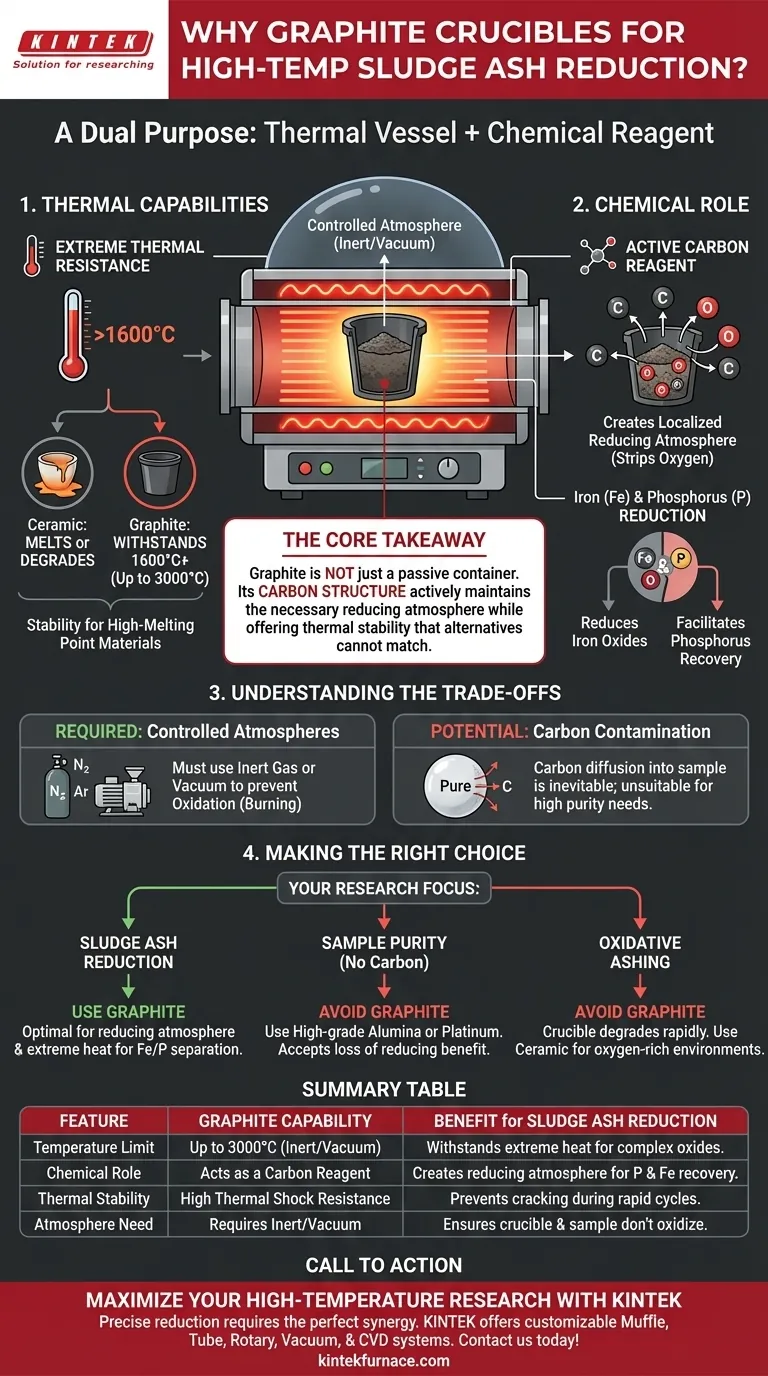
Graphite crucibles are indispensable for high-temperature sludge ash reduction because they serve a dual purpose: they function as both a highly heat-resistant vessel and an active chemical participant in the reduction process. By withstanding temperatures exceeding 1600°C and providing a source of carbon, they stabilize the thermal environment while directly facilitating the chemical transformation of the ash.
The Core Takeaway In sludge ash reduction, the crucible is not just a passive container; it is a reagent. Graphite is required because its carbon structure actively maintains the necessary reducing atmosphere, aiding in the separation of phosphorus and iron, while simultaneously offering thermal stability that ceramic or metal alternatives cannot match.

Thermal Capabilities in Extreme Environments
Withstanding Temperatures Beyond 1600°C
Standard laboratory ceramics often degrade or melt under the intense heat required for ash reduction. Graphite crucibles possess excellent thermal resistance, maintaining structural integrity well beyond the 1600°C threshold often required for these experiments.
Stability for High-Melting Point Materials
Sludge ash contains complex oxides that require significant energy to break down. Graphite is capable of remaining stable at temperatures exceeding 2000°C, and even up to 3000°C in vacuum environments. This ensures the vessel does not fail before the sample has fully reacted.
The Chemical Role: Graphite as a Reagent
Creating a Localized Reducing Atmosphere
The defining characteristic of a graphite crucible is that it is composed of carbon. During heating, the crucible material itself exhibits reductive properties. This effectively creates a reducing micro-environment around the sample, stripping oxygen from the sludge ash.
Facilitating Iron and Phosphorus Reduction
Sludge ash is frequently high in iron and phosphorus content. The graphite interface participates in the reaction, helping to reduce iron oxides. Furthermore, this carbon contact is critical for creating the specific atmospheric conditions necessary to successfully reduce phosphorus, allowing for its recovery or separation.
Understanding the Trade-offs
The Requirement for Controlled Atmospheres
Graphite's affinity for oxygen is a double-edged sword. While it aids reduction, the crucible itself will oxidize (burn) if exposed to standard air at high temperatures. Therefore, these experiments must occur within a tube furnace under an inert atmosphere (like Nitrogen or Argon) or a vacuum to prevent the crucible from disintegrating.
Potential for Carbon Contamination
Because the crucible participates in the reaction, carbon diffusion into the sample is inevitable. While this is desired for reduction experiments, it makes graphite unsuitable for processes requiring high-purity samples where carbon introduction would be considered a contaminant.
Making the Right Choice for Your Experiment
If your primary focus is Sludge Ash Reduction:
- Use Graphite: It is the optimal choice to ensure the necessary reducing atmosphere for separating iron and phosphorus while surviving extreme heat.
If your primary focus is Sample Purity (No Carbon):
- Avoid Graphite: Opt for high-grade Alumina or Platinum crucibles, accepting that you will lose the inherent reducing benefits of the vessel.
If your primary focus is Oxidative Ashing:
- Avoid Graphite: The crucible will degrade rapidly; use ceramic vessels designed for oxygen-rich environments.
Graphite is not merely a vessel; it is a critical component of the chemical equation that drives the reduction process to completion.
Summary Table:
| Feature | Graphite Crucible Capability | Benefit for Sludge Ash Reduction |
|---|---|---|
| Temperature Limit | Up to 3000°C (Inert/Vacuum) | Withstands extreme heat needed to break down complex oxides. |
| Chemical Role | Acts as a Carbon Reagent | Creates the reducing atmosphere required to recover phosphorus and iron. |
| Thermal Stability | High Thermal Shock Resistance | Prevents vessel cracking or failure during rapid high-temp cycles. |
| Atmosphere Need | Requires Inert/Vacuum | Ensures the crucible and sample do not oxidize prematurely. |
Maximize Your High-Temperature Research with KINTEK
Precise sludge ash reduction requires the perfect synergy between your reaction vessel and your furnace. KINTEK provides the high-performance equipment you need to achieve repeatable, accurate results. Backed by expert R&D and world-class manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique laboratory requirements.
Whether you are scaling up phosphorus recovery or refining material purity, our team is ready to provide the thermal solutions your research demands. Contact KINTEK today to discuss your custom furnace needs and see how our expertise can drive your next breakthrough.
Visual Guide
References
- Antoinette Kotzé, Sander Arnout. Thermochemical evaluation of elemental phosphorus recovery from sewage sludge. DOI: 10.17159/2411-9717/3556/2025
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What are the functions of silicon nitride ceramic sample holders? Precision for High-Temperature Levitation
- What is the function of a laboratory electric thermostatic drying oven in ZIF-8/ZIF-67 prep? Ensure MOF Integrity
- Why are ceramic containers with refractory clay seals utilized during the non-oxidative sintering of nickel composites?
- Why are lidded alumina crucibles required for LLZO sintering? Ensure High Ionic Conductivity and Phase Purity
- Why are high-purity alumina crucibles preferred over quartz crucibles at 1873 K? Ensure Precision at Extreme Heat
- How does excessive gas purging rate affect the alumina furnace tube? Prevent Cracking and Extend Tube Life
- What is the function of high-purity alumina crucibles? Protect Samples and Furnaces During Oxide Calcination
- Why is a high vacuum pumping system required for Bi2Se3-Nd2Se3 alloying? Ensure Purity in Rare Earth Synthesis



















