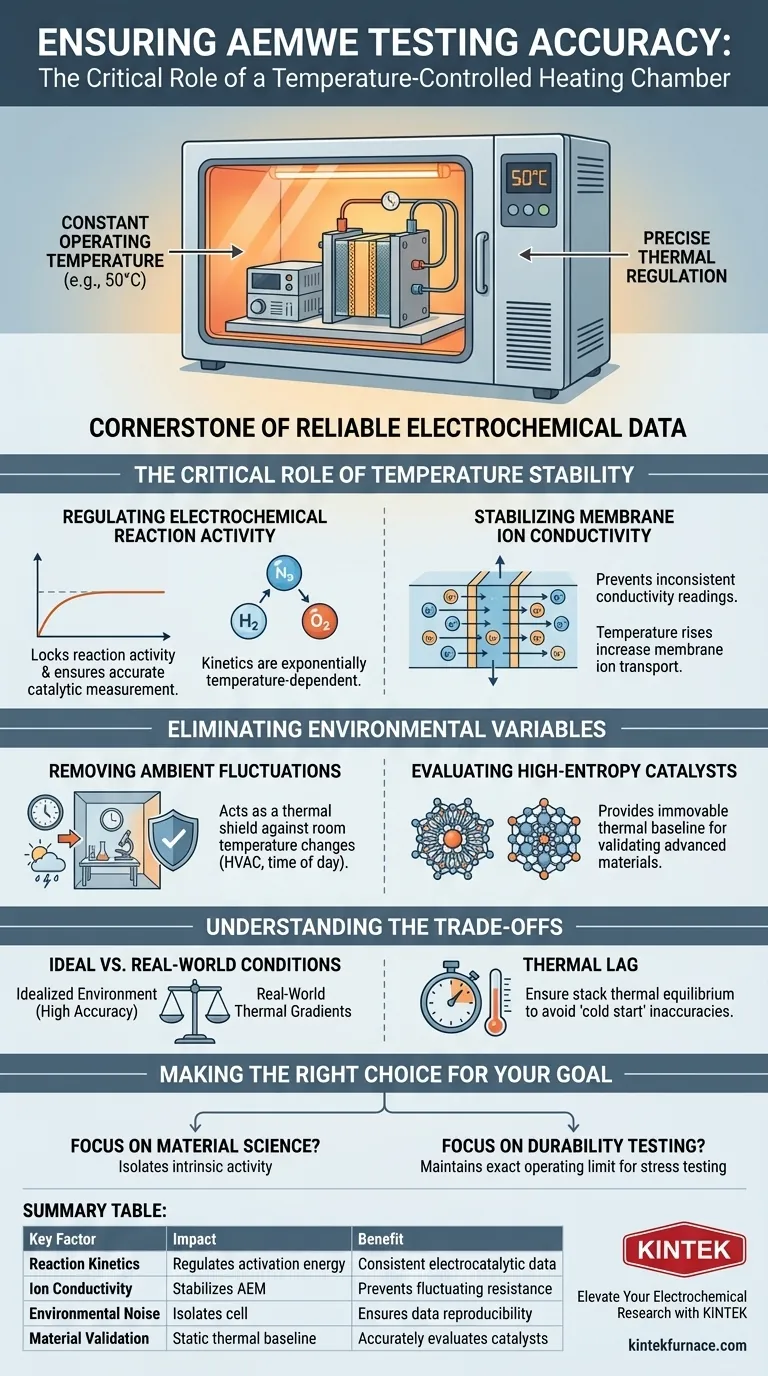
Precise thermal regulation is the cornerstone of reliable electrochemical data. A temperature-controlled heating chamber ensures the accuracy of Anion Exchange Membrane Water Electrolyzer (AEMWE) testing by strictly maintaining a constant operating temperature, such as 50 °C. This isolation eliminates external environmental interference, ensuring that any variation in performance is attributed to the cell components rather than ambient fluctuations.
By creating a stable thermal environment, the heating chamber neutralizes the impact of ambient temperature shifts. This ensures that observed changes in current or voltage are genuinely caused by the electrocatalytic performance and durability of the materials, not by thermal noise.

The Critical Role of Temperature Stability
Regulating Electrochemical Reaction Activity
Electrochemical kinetics are exponentially dependent on temperature. A slight increase in heat significantly lowers the activation energy required for the water splitting reaction.
If the temperature is allowed to drift, the reaction activity will fluctuate wildly. The heating chamber locks this variable, ensuring that the measured catalytic activity is accurate.
Stabilizing Membrane Ion Conductivity
The Anion Exchange Membrane (AEM) relies on thermal energy to facilitate the transport of ions.
As temperature rises, membrane ion conductivity typically increases, reducing internal resistance. Without a heating chamber to maintain a set point (e.g., 50 °C), conductivity readings would be inconsistent, distorting the overall efficiency data.
Eliminating Environmental Variables
Removing Ambient Fluctuations
Laboratory environments are rarely static; room temperature can change due to HVAC cycles or time of day.
A heating chamber acts as a thermal shield. It eliminates fluctuations caused by the surrounding environment, ensuring that the data collected at 9:00 AM is comparable to data collected at 5:00 PM.
Evaluating High-Entropy Catalysts
Advanced materials, such as high-entropy catalysts, require precise conditions to validate their performance.
To reliably evaluate the electrocatalytic performance of these complex materials, the thermal baseline must be immovable. This allows researchers to isolate the intrinsic properties of the catalyst from external noise.
Understanding the Trade-offs
Ideal vs. Real-World Conditions
While a heating chamber ensures high accuracy for research, it represents an idealized environment.
Real-world commercial electrolyzers may be exposed to thermal gradients that a uniform heating chamber does not simulate. It is important to acknowledge that lab results represent "best-case" stability.
Thermal Lag
There can be a delay between the chamber reaching 50 °C and the core of the stack reaching that same temperature.
Operators must ensure the stack has reached thermal equilibrium before recording data to avoid "cold start" inaccuracies.
Making the Right Choice for Your Goal
To maximize the value of your AEMWE testing, align your approach with your specific objectives:
- If your primary focus is Material Science: Use the chamber to lock temperature precisely; this isolates the intrinsic activity of your high-entropy catalysts.
- If your primary focus is Durability Testing: Maintain the chamber at the exact operating limit (e.g., 50 °C) to prove the membrane can withstand sustained thermal stress without degradation.
Accuracy in AEMWE testing is not just about measuring the right numbers, but about controlling the variables that create them.
Summary Table:
| Key Factor | Impact of Temperature Control | Benefit to AEMWE Testing |
|---|---|---|
| Reaction Kinetics | Regulates activation energy levels | Ensures consistent electrocatalytic activity measurements |
| Ion Conductivity | Stabilizes the Anion Exchange Membrane | Prevents fluctuating resistance and efficiency data |
| Environmental Noise | Isolates cell from ambient room changes | Ensures data reproducibility regardless of lab conditions |
| Material Validation | Provides a static thermal baseline | Accurately evaluates high-entropy catalyst performance |
Elevate Your Electrochemical Research with KINTEK
Precision is non-negotiable when testing next-generation AEMWE systems. KINTEK provides high-performance thermal solutions designed to eliminate variables and deliver repeatable results. Backed by expert R&D and world-class manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as specialized lab high-temp furnaces—all fully customizable to meet your unique electrolysis and material science requirements.
Ready to stabilize your testing environment? Contact KINTEK today to discuss your custom furnace needs
Visual Guide
References
- Chiung-Wen Chang, Shih‐Yuan Lu. High performance anion exchange membrane water electrolysis driven by atomic scale synergy of non-precious high entropy catalysts. DOI: 10.20517/energymater.2025.05
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What is the primary function of a high vacuum drying oven in B4C/Al powder pretreatment? Protect Purity & Prevent Pores
- How does a graphite furnace work? Achieve Ultra-Trace Element Analysis
- How are heat treatment furnaces utilized in the automotive industry? Enhance Component Durability and Performance
- Why is a constant temperature drying oven necessary during the preparation of porous activated carbon? Key Benefits
- How does glass frit function in SiOC coatings? Enhance Barrier Density with Liquid-Phase Healing
- Importance of NaH2PO2 Layout in V-Ni3S2/NF Phosphorization: Ensuring Uniform 3D Doping
- What role does a laboratory blast drying oven play in metal powder preparation? Ensure Purity & Prevent Oxidation
- Why is a drying oven with precise temperature control necessary for NiO-CGO anode supports? Ensure Cell Integrity



















