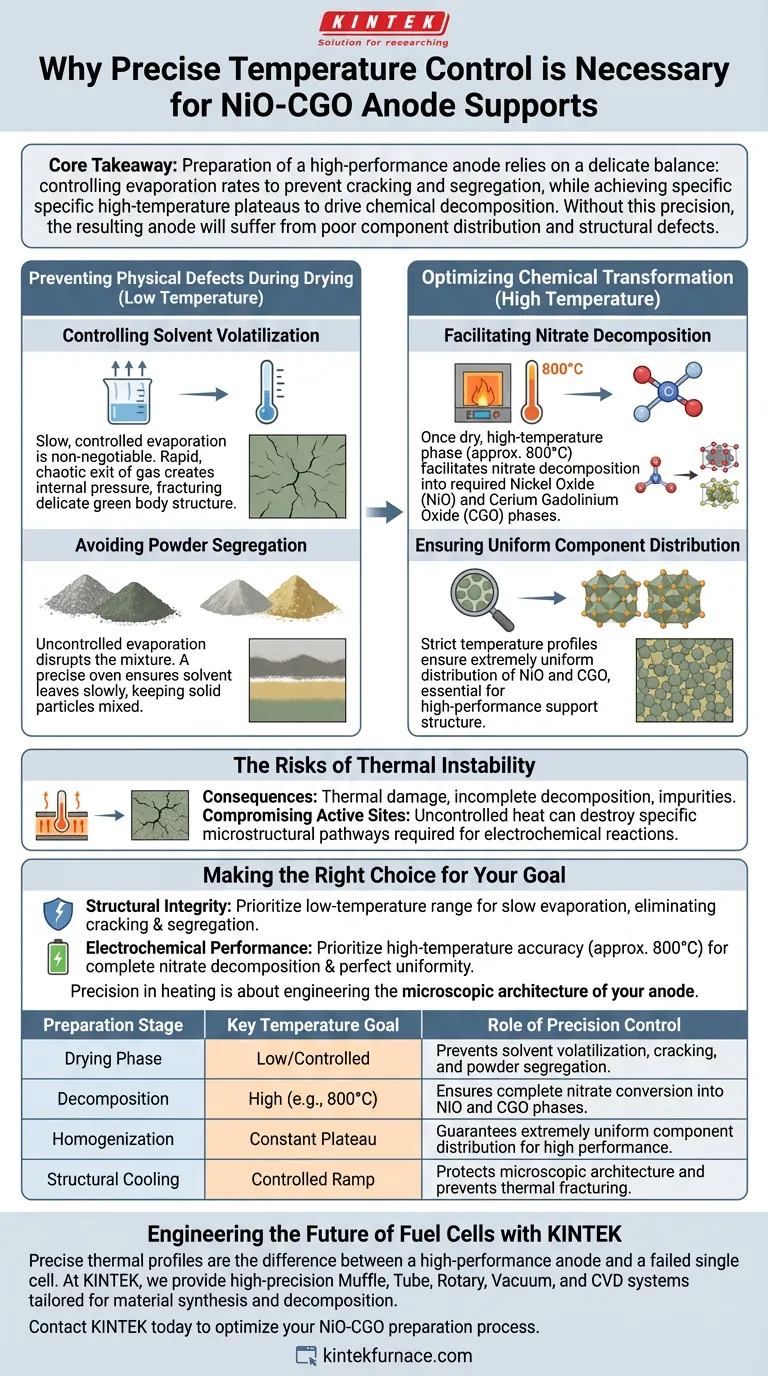
Precise temperature control is the fundamental requirement for ensuring the structural integrity and chemical homogeneity of NiO-CGO anode supports. It acts as the regulating mechanism that prevents physical destruction caused by rapid solvent volatilization and ensures the complete, uniform decomposition of nitrate precursors into the final active oxide phase.
Core Takeaway The preparation of a high-performance anode relies on a delicate balance: controlling evaporation rates to prevent cracking and segregation, while achieving specific high-temperature plateaus to drive chemical decomposition. Without this precision, the resulting anode will suffer from poor component distribution and structural defects.
Preventing Physical Defects During Drying
Controlling Solvent Volatilization
The initial stage of preparing NiO-CGO supports involves removing moisture from the solution. Slow, controlled evaporation is non-negotiable here.
If the temperature rises too quickly or fluctuates, the solvent will volatilize rapidly. This chaotic exit of gas creates internal pressure that fractures the delicate green body structure.
Avoiding Powder Segregation
Rapid drying does more than just crack the material; it disrupts the mixture itself.
Uncontrolled evaporation leads to powder segregation, where the nickel and cerium components separate rather than staying mixed. A precise oven ensures the solvent leaves slowly enough to leave the solid particles exactly where they are intended to be.
Optimizing Chemical Transformation
Facilitating Nitrate Decomposition
Once the material is dry, the process moves to a high-temperature phase, typically reaching 800 degrees Celsius.
At this stage, the goal shifts from physical drying to chemical conversion. The equipment must hold steady temperatures to facilitate the decomposition of nitrates. This converts the raw precursors into the required Nickel Oxide (NiO) and Cerium Gadolinium Oxide (CGO) phases.
Ensuring Uniform Component Distribution
The ultimate goal of this thermal treatment is homogeneity.
By maintaining strict temperature profiles, you ensure an extremely uniform distribution of NiO and CGO throughout the composite. This uniformity is what allows the anode to function effectively as a high-performance support structure in the final fuel cell.
The Risks of Thermal Instability
Consequences of Inconsistency
Using equipment without precise control introduces variables that ruin reproducibility.
If the temperature overshoots or unevenly heats the batch, you risk thermal damage to the material structure. Conversely, undershooting results in incomplete decomposition, leaving behind impurities that degrade cell performance.
Compromising Active Sites
While the primary goal is oxide formation, the principle of protecting the material integrity remains.
Just as with general adsorbents, uncontrolled heat can destroy the specific structural arrangement needed for the material to function. In the context of NiO-CGO, this manifests as a loss of the specific microstructural pathways required for electrochemical reactions.
Making the Right Choice for Your Goal
To achieve a viable single cell, you must tailor your thermal profile to the specific stage of preparation.
- If your primary focus is Structural Integrity: Prioritize the low-temperature range to ensure slow evaporation, which eliminates the risk of cracking and macroscopic segregation.
- If your primary focus is Electrochemical Performance: Prioritize the high-temperature accuracy (around 800°C) to guarantee complete nitrate decomposition and perfect uniformity of the active NiO and CGO phases.
Precision in heating is not just about drying; it is about engineering the microscopic architecture of your anode.
Summary Table:
| Preparation Stage | Key Temperature Goal | Role of Precision Control |
|---|---|---|
| Drying Phase | Low/Controlled | Prevents solvent volatilization, cracking, and powder segregation. |
| Decomposition | High (e.g., 800°C) | Ensures complete nitrate conversion into NiO and CGO phases. |
| Homogenization | Constant Plateau | Guarantees extremely uniform component distribution for high performance. |
| Structural Cooling | Controlled Ramp | Protects the microscopic architecture and prevents thermal fracturing. |
Engineering the Future of Fuel Cells with KINTEK
Precise thermal profiles are the difference between a high-performance anode and a failed single cell. At KINTEK, we understand that your research demands absolute consistency. Backed by expert R&D and world-class manufacturing, we provide high-precision Muffle, Tube, Rotary, Vacuum, and CVD systems tailored specifically for the rigorous requirements of material synthesis and chemical decomposition.
Whether you need delicate moisture removal or 800°C+ oxide conversion, our customizable lab high-temperature furnaces deliver the thermal stability you need to prevent segregation and ensure material homogeneity. Contact KINTEK today to optimize your NiO-CGO preparation process.
Visual Guide
References
- Paula Rosendo, Daniel Muñoz‐Gil. Optimisation of the electrochemical performance of (Nd,Gd)<sub>1/3</sub>Sr<sub>2/3</sub>CoO<sub>3−<i>δ</i></sub> cathode for solid oxide fuel cells <i>via</i> spray-pyrolysis deposition and decoration with Ag nanoparticles. DOI: 10.1039/d3ta05917k
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What advantages does AlMe2iPrO (DMAI) offer over Trimethylaluminum (TMA)? Achieve Superior Area Selectivity
- What is zirconium dioxide and how is it stabilized for dental use? Discover the Science Behind Durable Dental Ceramics
- What occurs during the recrystallization stage of annealing? Restore Ductility and Reset Microstructure
- How does a Zinc Oxide (ZnO) catalyst affect PET pyrolysis? Optimize Yields & Efficiency
- Why is a vacuum desiccator used for the preservation of extracted fruit peel extracts? Protect Bioactive Compounds
- What is the primary function of a high-temperature sintering furnace operating at 1173 K in the preparation of porous oxide precursors? Achieve Structural Integrity for Your Precursors
- How does the precise control of heating rates affect sewage sludge biochar? Master Stability & Metal Stabilization
- How does the temperature field provided by a High-Temperature Reaction Furnace promote pore development? 700-800°C Mastery



















