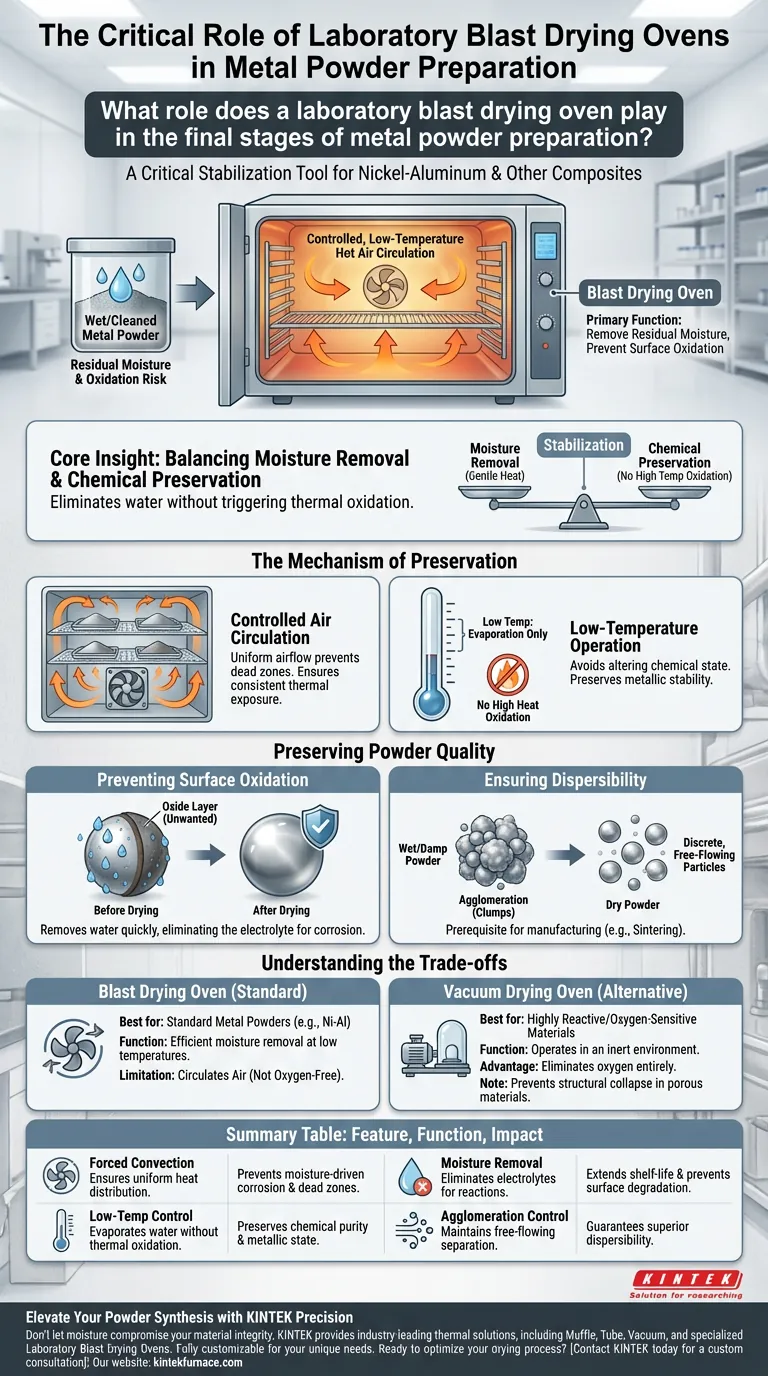
The laboratory blast drying oven serves as a critical stabilization tool in the final stages of preparing metal powders, particularly nickel-aluminum composites. Its primary function is to remove residual moisture from cleaned powders using a controlled, low-temperature hot air circulation system to prevent surface oxidation and ensure the physical quality of the material.
Core Insight: The drying process is a delicate balance between moisture removal and chemical preservation. By utilizing gentle, circulating heat, the blast drying oven eliminates water that promotes corrosion without subjecting the active metal surface to temperatures that would trigger oxidation.
The Mechanism of Preservation
Controlled Air Circulation
The oven operates by "blasting" or circulating heated air throughout the chamber. This forced convection ensures that every particle of the metal powder is exposed to a consistent thermal environment.
Uniform airflow is essential to prevent "dead zones" where moisture could linger. Even trace amounts of remaining moisture can act as a catalyst for degradation in active metals.
Low-Temperature Operation
Contrary to high-temperature sintering or annealing, this stage focuses on gentle treatment. The oven operates at low temperatures specifically to avoid altering the chemical state of the metal.
High heat accelerates oxidation reactions. By keeping the temperature low, the process removes water through evaporation while keeping the metal chemically stable.
Preserving Powder Quality
Preventing Surface Oxidation
The most significant risk during the drying of active metal powders (such as nickel-aluminum) is oxidation. Water and oxygen can react with the metal surface to form unwanted oxide layers.
The blast drying oven mitigates this by removing the water quickly and efficiently. By eliminating the electrolyte (water) needed for electrochemical corrosion, the oven preserves the metallic purity of the powder.
Ensuring Dispersibility
Beyond chemical purity, the physical state of the powder is paramount. Wet or damp powders tend to agglomerate, forming clumps that are difficult to separate later.
Thorough drying ensures the final product consists of discrete, free-flowing particles. This "dispersibility" is a prerequisite for any subsequent manufacturing processes, such as mixing, pressing, or sintering.
Understanding the Trade-offs
The Oxygen Limitation
It is vital to recognize that a blast drying oven circulates air. While low temperatures minimize reaction risks for metals like nickel-aluminum, the environment is not oxygen-free.
For materials that are extremely sensitive to oxygen even at low temperatures, or for processes requiring the removal of organic solvents, a vacuum drying oven is often the superior choice. Vacuum ovens operate in an inert environment, preventing oxidation more aggressively than blast ovens.
Moisture vs. Structure
Rapid heating can sometimes cause steam to release too quickly, damaging the internal structure of porous materials.
While this is more critical in organic materials (like biochar), the principle applies to porous metal powders as well. The controlled nature of the blast oven prevents structural collapse caused by rapid evaporation.
Making the Right Choice for Your Goal
To ensure the integrity of your final metal powder, apply the following selection criteria:
- If your primary focus is standard metal powders (e.g., Ni-Al): Use a blast drying oven at low temperatures to efficiently remove moisture while maintaining surface quality.
- If your primary focus is highly reactive or oxygen-sensitive materials: Consider a vacuum drying oven to eliminate oxygen entirely during the heating process.
- If your primary focus is preventing agglomeration: Ensure the drying cycle is sufficiently long to remove all physically adsorbed water, guaranteeing a free-flowing powder.
The drying phase is not merely a cleaning step; it is a preservation strategy that defines the shelf-life and usability of your synthesized material.
Summary Table:
| Feature | Function in Metal Powder Drying | Impact on Material Quality |
|---|---|---|
| Forced Convection | Ensures uniform heat distribution across all particles | Prevents moisture-driven corrosion and dead zones |
| Low-Temp Control | Evaporates water without triggering thermal oxidation | Preserves chemical purity and metallic state |
| Moisture Removal | Eliminates electrolytes required for electrochemical reactions | Extends shelf-life and prevents surface degradation |
| Agglomeration Control | Maintains discrete, free-flowing particle separation | Guarantees superior dispersibility for manufacturing |
Elevate Your Powder Synthesis with KINTEK Precision
Don't let moisture compromise your material integrity. KINTEK provides industry-leading thermal solutions, including Muffle, Tube, Vacuum, and specialized Laboratory Blast Drying ovens designed to meet the rigorous demands of metal powder preparation. Backed by expert R&D and manufacturing, our systems are fully customizable to your unique thermal processing needs, ensuring your powders remain pure, stable, and free-flowing.
Ready to optimize your drying process? Contact KINTEK today for a custom consultation!
Visual Guide
References
- Gülizar Sarıyer, H. Erdem Çamurlu. Production and Characterization of Ni0.50 Al0.50 and Ni0.55 Al0.45 Powders by Volume Combustion Synthesis. DOI: 10.17776/csj.1280582
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
People Also Ask
- Why are deoxidizer powders sealed inside iron bolts? Achieve Precise Chemical Control in Steel Inclusion Preparation
- What is the significance of industrial drying equipment for metal powders? Master Post-Processing & Quality Control
- What is the use of furnace in laboratory? Unlock Precise High-Temperature Control for Material Transformations
- What is the purpose of the annealing process in OLED preparation? Optimize Film Stability and Device Efficiency
- How does a Bridgman furnace control single-crystal quality? Master Precision Directional Solidification
- What are the limitations of functional group grafting through high-temperature heating? Achieve Chemical Precision
- Why is a constant temperature and humidity curing chamber essential for geopolymerization? Ensure Structural Strength
- What are some drawbacks of electric heating methods? High Costs and Grid Dependence Explained



















