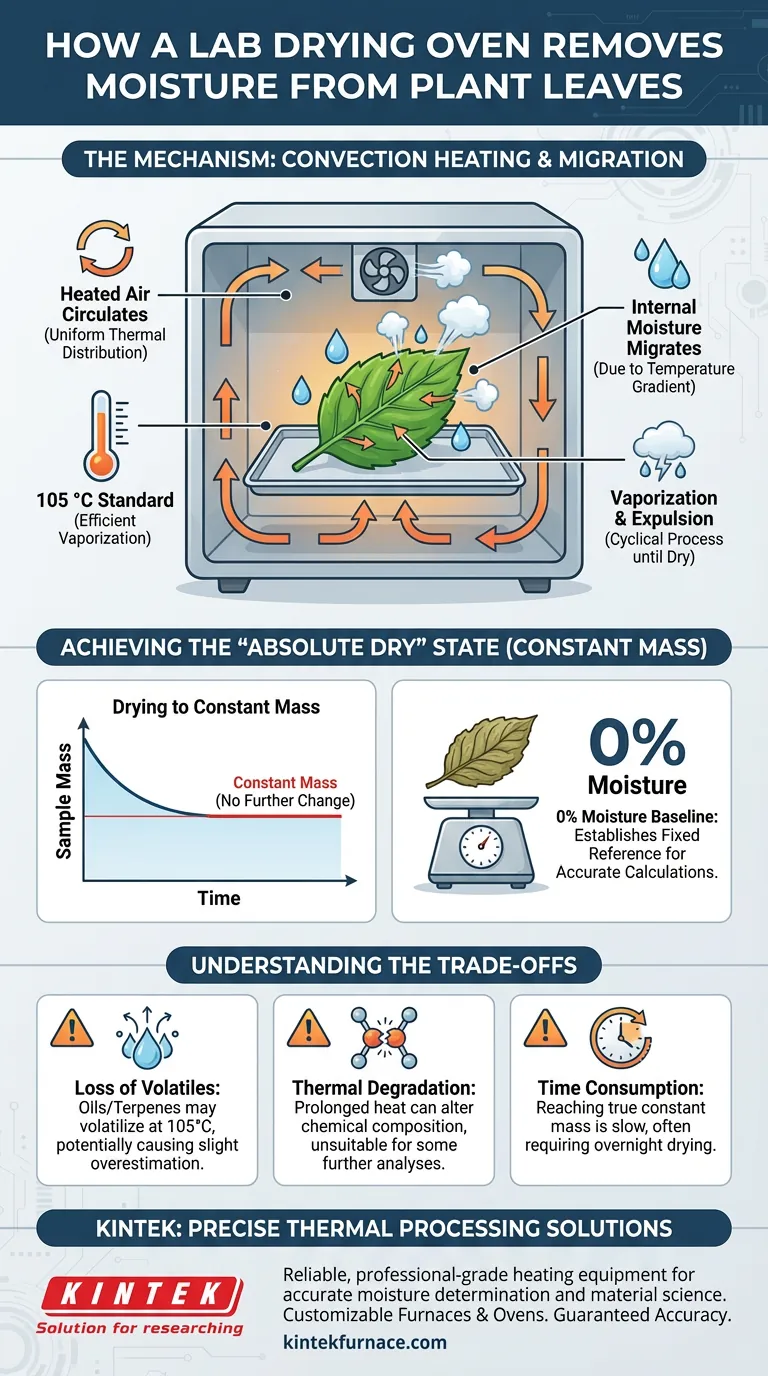
A laboratory drying oven utilizes convection heating to create a stable, high-temperature environment that drives moisture out of plant leaf samples. By maintaining a constant temperature, typically at 105 °C, the oven forces internal water to migrate to the leaf surface where it vaporizes and is expelled until the sample reaches a completely dry state.
The drying oven ensures accurate moisture content determination by establishing an "absolute dry" baseline. By heating samples until their mass ceases to change, it completely removes free and bound water, providing the fixed reference point required for precise dry-basis calculations.

The Mechanism of Moisture Removal
To understand how a drying oven facilitates analysis, one must look at the thermodynamic process occurring within the leaf tissue.
Convection Heating Principles
The oven operates on the principle of convection heating. Heated air circulates around the sample, ensuring uniform thermal energy distribution.
This consistent heat transfer is critical. It prevents "hot spots" that could burn the sample while ensuring other areas remain moist.
Internal Moisture Migration
The external heat creates a temperature gradient within the leaf structure. This stimulates the continuous migration of internal moisture from the core of the sample toward the outer surfaces.
Vaporization and Expulsion
Once moisture reaches the surface of the leaf, the high temperature causes it to vaporize.
The circulating air then expels this water vapor from the chamber. This process continues cyclically until the water supply within the cellular structure is exhausted.
Establishing the "Absolute Dry" State
The ultimate goal of using a drying oven is not just to dry the sample, but to reach a specific analytical state known as "constant mass."
The 105 °C Standard
Laboratories typically set the oven to 105 °C. This temperature is slightly above the boiling point of water, ensuring efficient vaporization without causing immediate combustion of the plant material.
Drying to Constant Mass
The process is complete only when the mass of the sample no longer changes.
This indicates that all evaporable water has been removed. Whether it takes a few hours or up to 72 hours (as seen with denser materials like wood), this state represents 0% moisture content.
Creating a Calculation Baseline
Reaching this absolute dry state provides a mathematically solid baseline.
Once the dry weight is confirmed, you can accurately calculate the original moisture content. It also allows researchers to adjust samples to specific moisture levels (e.g., 10% or 20%) for subsequent experiments, as the dry mass is a known, unchanging variable.
Understanding the Trade-offs
While drying ovens are the standard for moisture determination, the method relies on aggressive thermal treatment which introduces specific variables.
Loss of Volatiles
The primary limitation of this method is that it does not distinguish between water and other volatile compounds.
At 105 °C, certain oils or terpenes in the plant leaf may also volatilize. This can result in a slight overestimation of moisture content, as the weight loss is assumed to be water alone.
Thermal Degradation risks
Prolonged exposure to high heat can alter the chemical composition of the sample.
If the sample is needed for further chemical analysis (beyond simple moisture content), the heat may degrade sensitive compounds.
Time Consumption
Achieving a true "constant mass" is a slow process. Unlike rapid moisture analyzers, a standard oven method often requires overnight drying or longer to ensure deep-bound water is fully evacuated.
Ensuring Precision in Your Analysis
To derive the most accurate data from your drying oven, align your method with your specific analytical goals.
- If your primary focus is Absolute Accuracy: Ensure you dry the sample until the weight creates a flatline (constant mass), regardless of the time required.
- If your primary focus is Sample Preservation: Be aware that the 105 °C standard destroys biological activity; this method is strictly for gravimetric analysis, not for preserving tissue viability.
Reliable data depends on the certainty that the final weight represents the sample's structure alone, stripped of all variable water content.
Summary Table:
| Process Stage | Mechanism | Key Outcome |
|---|---|---|
| Heating | Convection heating at 105 °C | Uniform thermal distribution without burning |
| Migration | Temperature gradient driving | Internal moisture moves to the leaf surface |
| Vaporization | Surface evaporation | Water vapor is expelled from the chamber |
| Final State | Drying to constant mass | Establishes a 0% moisture baseline for calculations |
Optimize Your Lab’s Thermal Processing with KINTEK
Precise moisture determination requires the steady, reliable performance of professional-grade heating equipment. KINTEK provides high-performance laboratory drying ovens and specialized furnace systems designed to meet the rigorous demands of material science and biological research.
Our Value to You:
- Expert R&D & Manufacturing: Backed by industry-leading engineering for superior temperature uniformity.
- Customizable Solutions: From Muffle, Tube, and Rotary Furnaces to Vacuum and CVD systems, we tailor equipment to your unique lab specifications.
- Guaranteed Accuracy: Achieve constant mass faster with advanced airflow and thermal control.
Ready to elevate your analytical precision? Contact KINTEK today for a consultation.
Visual Guide
References
- Effects of Drying Temperatures on Nutritional and Phytochemical Properties of Gongronema Latifolium Leaves. DOI: 10.63958/azojete/2025/21/2/001
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Small Rotary Kiln Calciner
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
People Also Ask
- What materials are commonly used in the construction of a muffle furnace? Discover Durable Components for High-Temp Labs
- Why is a precision muffle furnace required for TiO2 sintering? Optimize Your Dye-Sensitized Solar Cell Performance
- What fire safety equipment should be available when using a benchtop furnace? Essential Gear for Lab Safety
- What temperature range can muffle furnaces typically operate within? Find the Perfect Fit for Your Lab
- What is a digital muffle furnace and what are its primary functions? Achieve Pure, High-Temperature Processing
- Why is a muffle furnace essential for the combustion step in the preparation of perovskite catalysts?
- What role does a muffle furnace play in evaluating the oxidation resistance of WC-Fe-Ni-Co? Material Testing Insights
- What are the limitations of muffle furnaces in industrial applications? Uncover Key Constraints for Smart Lab Choices

















