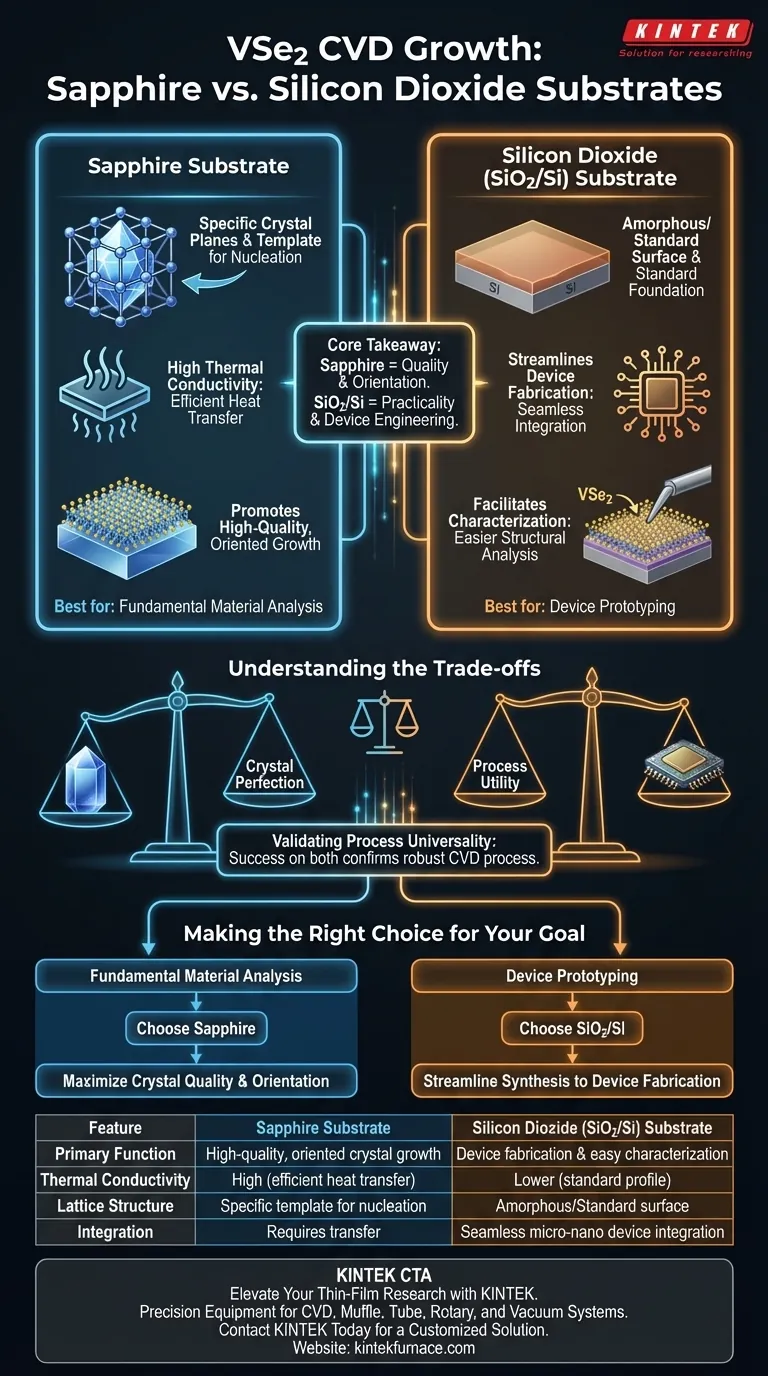
Sapphire substrates are utilized primarily to promote high-quality, oriented crystal growth due to their high thermal conductivity and specific lattice structures. Conversely, silicon dioxide (SiO2/Si) substrates are selected to facilitate seamless integration into micro-nano device fabrication and to allow for easier structural characterization.
Core Takeaway: While sapphire optimizes the physical quality and orientation of VSe2 crystals during synthesis, SiO2/Si is favored for its practical utility in downstream device engineering. Successfully growing VSe2 on both substrate types serves to validate the universality and robustness of the CVD growth process.

The Role of Sapphire in Crystal Synthesis
Leveraging Thermal Properties
Sapphire substrates are characterized by high thermal conductivity.
In a Chemical Vapor Deposition (CVD) environment, this property ensures efficient heat transfer, which is critical for maintaining the stable temperatures required for uniform material deposition.
Achieving Oriented Growth
The surface of a sapphire substrate presents specific crystal plane structures.
These structures act as a template, influencing nucleation and promoting the oriented growth of VSe2. This results in crystals of significantly higher quality compared to those grown on non-crystalline or mismatched surfaces.
The Utility of Silicon Dioxide (SiO2/Si)
Streamlining Device Fabrication
The primary function of SiO2/Si substrates in this context is compatibility.
Because SiO2/Si is the standard foundation for semiconductor technology, growing VSe2 directly on this substrate simplifies the subsequent fabrication of micro-nano devices. It eliminates complex transfer processes often required when moving crystals from a growth substrate to a device substrate.
Facilitating Characterization
SiO2/Si substrates are specifically noted for aiding in structural characterization.
The properties of the substrate make it easier for researchers to analyze the physical structure of the deposited VSe2 material, ensuring that the synthesized layers meet technical specifications.
Understanding the Trade-offs
Quality vs. Applicability
The choice between these substrates represents a trade-off between crystal perfection and process utility.
Sapphire is the superior choice when the primary metric is the intrinsic quality and alignment of the crystal lattice. However, SiO2/Si is superior when the end goal is the rapid development and testing of electronic devices.
Validating Process Universality
Using disparate substrates is not just about choosing one over the other; it is a method of process validation.
By demonstrating that VSe2 can be successfully grown on both the high-performance surface of sapphire and the practical surface of SiO2, researchers confirm that their CVD process is "universal" and not strictly dependent on a specific substrate interaction to work.
Making the Right Choice for Your Goal
To select the correct substrate for your specific VSe2 application, evaluate your immediate objectives:
- If your primary focus is fundamental material analysis: Choose sapphire to maximize crystal quality, orientation, and thermal management during growth.
- If your primary focus is device prototyping: Choose silicon dioxide (SiO2/Si) to streamline the transition from synthesis to micro-nano device fabrication and characterization.
By matching the substrate's functional strengths to your project's phase, you ensure efficiency in both research and application.
Summary Table:
| Feature | Sapphire Substrate | Silicon Dioxide (SiO2/Si) Substrate |
|---|---|---|
| Primary Function | High-quality, oriented crystal growth | Device fabrication & easy characterization |
| Thermal Conductivity | High (efficient heat transfer) | Lower (standard semiconductor profile) |
| Lattice Structure | Specific template for nucleation | Amorphous/Standard surface |
| Integration | Requires transfer for device use | Seamless micro-nano device integration |
| Best Used For | Fundamental material analysis | Rapid prototyping & device engineering |
Elevate Your Thin-Film Research with KINTEK
Precision in VSe2 synthesis starts with the right equipment and the right substrate. Whether you are targeting fundamental crystal analysis on sapphire or streamlining device fabrication on SiO2/Si, KINTEK provides the specialized tools to ensure success.
Backed by expert R&D and manufacturing, we offer high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to your unique research needs. Don't settle for inconsistent growth—partner with KINTEK to achieve professional-grade results in every synthesis cycle.
Contact KINTEK Today for a Customized Solution
Visual Guide
References
- Gangtae Jin. Controlled Vapor-Phase Synthesis of VSe2 via Selenium-Driven Gradual Transformation of Single-Crystalline V2O5 Nanosheets. DOI: 10.3390/nano15070548
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Ultra High Vacuum CF Flange Stainless Steel Sapphire Glass Observation Sight Window
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What challenges and limitations are associated with CVD? Overcome Key Constraints for Better Film Coating
- What are the different types of Chemical Vapor Deposition? Explore Key Methods for Thin Film Applications
- How are CVD furnaces used in material preparation? Essential for Thin Films & Nanomaterials
- Where is CVD used? Unlocking High-Performance Materials in Electronics, Energy & Aerospace
- What are the limitations or challenges of the CVD process? Understand Key Hurdles for Better Decisions
- How is CVD used in electronics manufacturing? Build High-Purity, Uniform Layers for Advanced Electronics
- What are intermetallic compounds, and how are they used in CVD? Unlock Advanced Thin Film Solutions
- What is Chemical Vapor Deposition (CVD) used for? Unlock High-Performance Thin Films for Your Applications



















