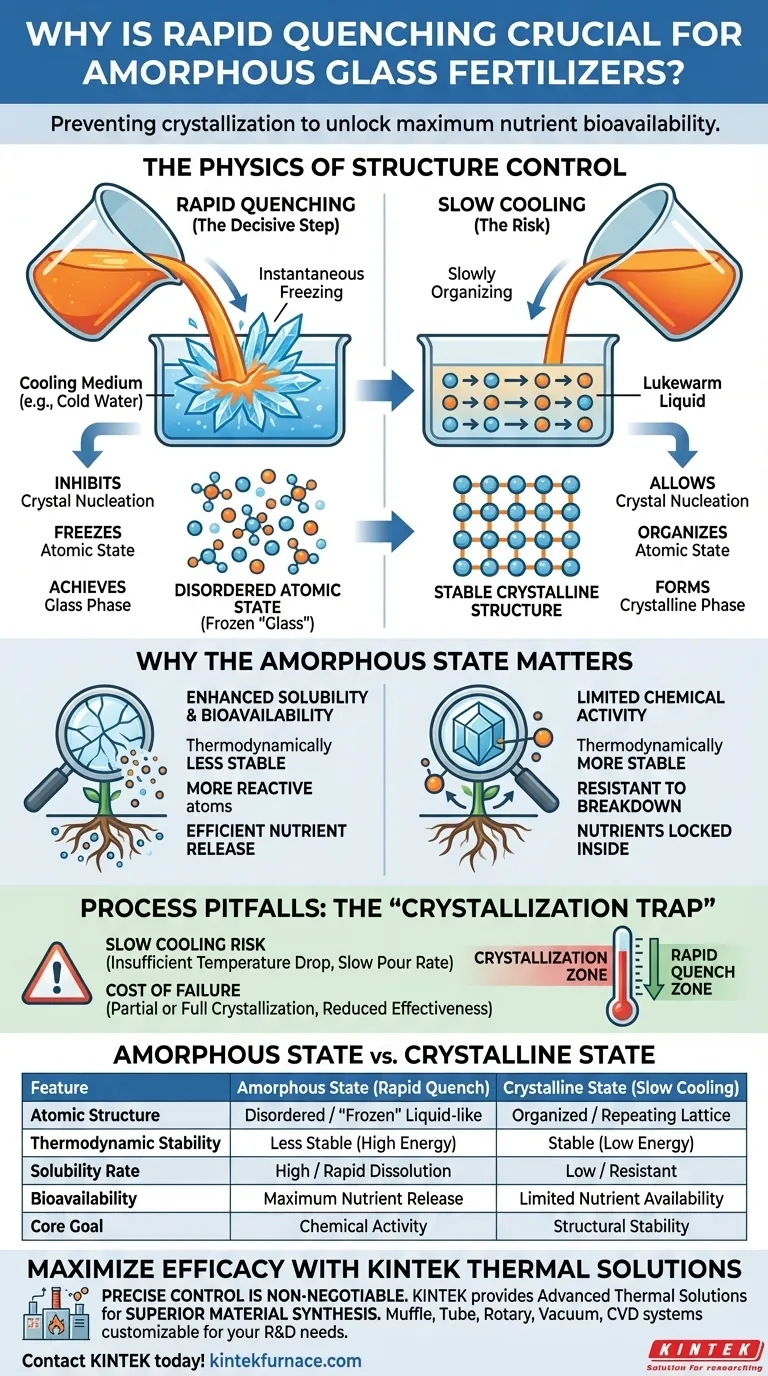
Rapid quenching is the decisive processing step required to prevent the fertilizer material from organizing into a stable crystalline structure. By pouring the molten mixture directly into a cooling medium, such as cold water, the temperature is lowered instantaneously. This extreme cooling rate physically freezes the atoms in a disordered state before they have time to form crystal nuclei or grow into defined crystals.
The primary objective of rapid quenching is to lock the material into a completely amorphous "glass" phase. This disordered atomic structure is essential because it significantly enhances the fertilizer's solubility and chemical activity, ensuring nutrients are readily available within the soil environment.
The Physics of Structure Control
Inhibiting Crystal Nucleation
In a standard cooling process, atoms naturally arrange themselves into organized, repeating patterns known as crystals.
Rapid quenching interrupts this natural thermodynamic process. The cooling rate is so high that the formation of "crystal nuclei"—the seeds from which crystals grow—is completely inhibited.
Freezing the Atomic State
The process relies on speed. You are essentially racing against the material's natural tendency to organize.
By instantly dropping the temperature, the atomic movement is arrested. The disordered, fluid-like atomic structure of the melt is "frozen" into a solid state without rearranging.
Achieving the Glass Phase
The result of this frozen disorder is a material known as a "glass."
While it feels solid to the touch, chemically and structurally, it resembles a liquid frozen in time. This is the definition of the amorphous state.
Why the Amorphous State Matters
Enhanced Solubility
The core problem with crystalline fertilizers is often their stability; they can be too resistant to breaking down.
The amorphous glass phase created by rapid quenching is thermodynamically less stable than a crystal. This instability allows the material to dissolve more easily when introduced to water or soil moisture.
Increased Chemical Activity
Beyond simple solubility, the amorphous structure boosts chemical activity.
Because the atoms are not locked in a rigid lattice, they are more reactive. This ensures that the nutrients within the glass matrix are released more efficiently into the soil environment.
The "Crystallization Trap": Process Pitfalls
The Risk of Slow Cooling
The cooling rate is a binary success factor: it is either fast enough, or it is not.
If the cooling medium (e.g., the water) is not cold enough, or if the pour rate is too slow, the material will spend time in a temperature zone where crystallization can occur.
The Cost of Failure
If the quench is insufficient, the material will partially or fully crystallize.
A crystalline fertilizer lacks the enhanced solubility of its amorphous counterpart. Failing to quench rapidly enough renders the fertilizer less effective, as the nutrients effectively remain locked inside the ordered crystal structure.
Making the Right Choice for Your Goal
To ensure the efficacy of glass fertilizers, the cooling process must be prioritized as a critical quality control point.
- If your primary focus is Maximum Bioavailability: Ensure the cooling medium provides an instantaneous temperature drop to guarantee a 100% amorphous structure.
- If your primary focus is Process Consistency: Monitor the quenching rate strictly; any deviation toward slower cooling will result in unwanted crystallization and reduced product performance.
Rapid quenching transforms a standard chemical mixture into a highly active, bio-available nutrient source.
Summary Table:
| Feature | Amorphous State (Rapid Quench) | Crystalline State (Slow Cooling) |
|---|---|---|
| Atomic Structure | Disordered / Liquid-like "Frozen" | Organized / Repeating Lattice |
| Thermodynamic Stability | Less Stable (High Energy) | Stable (Low Energy) |
| Solubility Rate | High / Rapid Dissolution | Low / Resistant to Breakdown |
| Bioavailability | Maximum Nutrient Release | Limited Nutrient Availability |
| Core Goal | Chemical Activity | Structural Stability |
Maximize Fertilizer Efficacy with KINTEK Advanced Thermal Solutions
Precise control over quenching and thermal states is non-negotiable when producing high-performance amorphous materials. KINTEK provides the cutting-edge tools necessary to achieve these exact parameters. Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, CVD systems, and other lab high-temp furnaces, all customizable for your unique research or production needs.
Whether you are optimizing cooling rates or developing next-generation nutrient delivery systems, our engineering team is ready to support your innovation. Contact KINTEK today to discuss your customization requirements and ensure your lab is equipped for superior material synthesis.
Visual Guide
References
- Anna Berezicka, Magdalena Szumera. Alteration of Sulfur-Bearing Silicate-Phosphate (Agri)Glasses in Soil Environment: Structural Characterization and Chemical Reactivity of Fertilizer Glasses: Insights from ‘In Vitro’ Studies. DOI: 10.3390/molecules30081684
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Ultra High Vacuum CF Observation Window Flange with High Borosilicate Glass Sight Glass
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What is the objective of placing TC4 titanium alloy parts on asbestos pads? Control Stress and Thermal Shock
- What is the primary function of a laboratory blast drying oven? Mastering Coconut Husk Biochar Preparation
- How does an electric furnace ensure accurate gasification? Master Isothermal and Dynamic Thermal Control
- What is a benchtop industrial oven? Maximize Space and Efficiency in Your Lab
- What are the process advantages of using PVT compared to solution methods for organic crystals? Enhance Purity & Uniformity
- How does a precision temperature-controlled heating furnace enhance medium-entropy alloys? Achieve Optimal Hardness
- What is the purpose of using a thermal evaporation coating system? Enhancing I-V Testing Accuracy for Nanocomposites
- Why is preheating a metal mold to 660 °C necessary for Al/Cu bimetallic composites? Unlock Strong Chemical Bonding



















