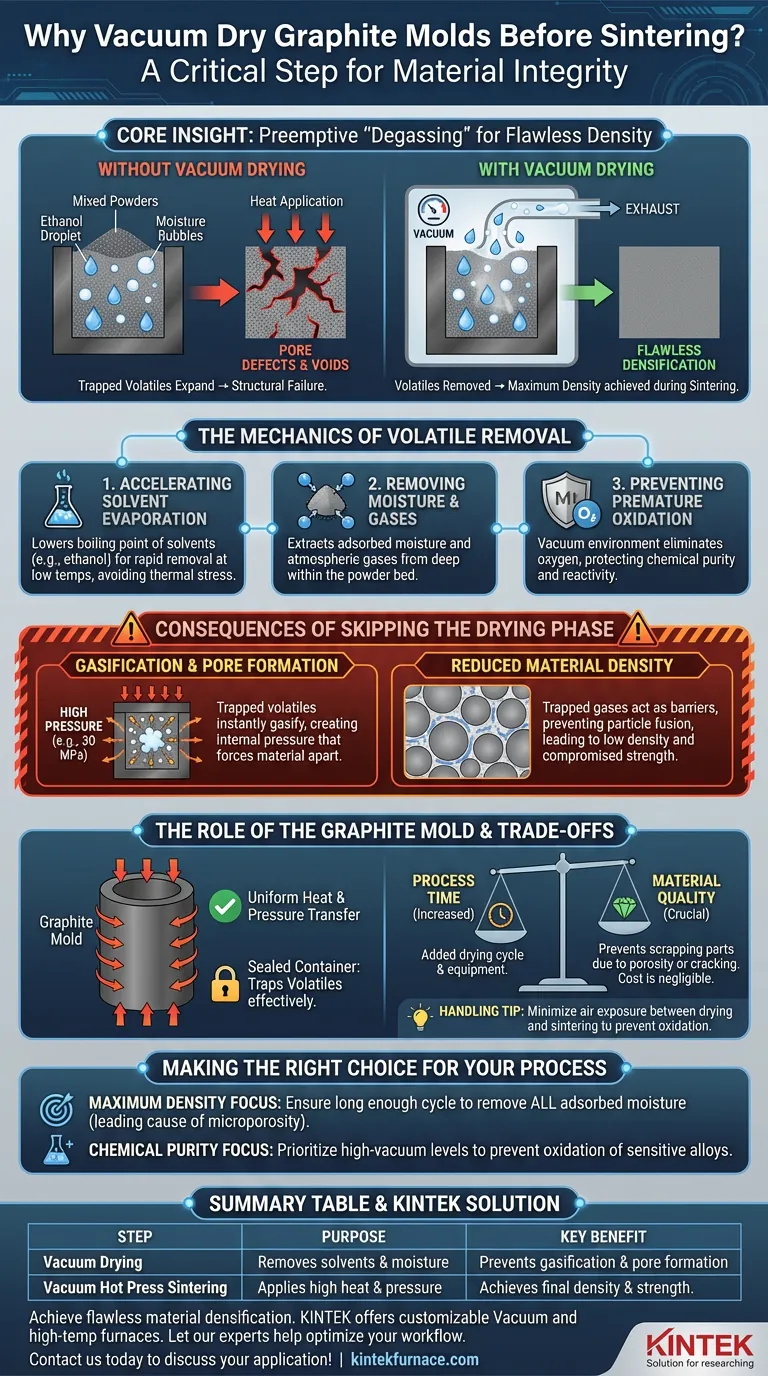
Treating graphite molds containing mixed powders in a vacuum drying oven is a non-negotiable step for ensuring material integrity. This process is strictly necessary to thoroughly remove residual solvents, such as ethanol, and adsorbed moisture introduced during the mixing phase. By performing this at low temperatures under a vacuum, you eliminate volatile components that would otherwise destroy the material's structure during high-temperature sintering.
Core Insight: The vacuum drying phase acts as a preemptive "degassing" stage. It ensures that when the material is eventually subjected to extreme heat and pressure, there are no trapped liquids or gases left to expand, thereby preventing the formation of internal voids and ensuring the final component achieves maximum density.

The Mechanics of Volatile Removal
Accelerating Solvent Evaporation
During the auxiliary mixing process, solvents like ethanol are often used to blend powders. A vacuum drying oven lowers the boiling point of these solvents. This allows for rapid and thorough evaporation at lower temperatures, ensuring the powder mixture is completely dry without subjecting it to thermal stress prematurely.
Removing Adsorbed Moisture and Gases
Beyond mixing solvents, metal powders naturally adsorb moisture and atmospheric gases. Vacuum drying extracts these contaminants from the deep recesses of the powder bed. This is critical because even trace amounts of moisture can react chemically at sintering temperatures, leading to material degradation.
Preventing Premature Oxidation
Heating metal powders in the presence of air or moisture can lead to immediate surface oxidation. The vacuum environment removes oxygen from the chamber while drying the powder. This protects the chemical purity of the metal powders, ensuring they remain reactive and ready for bonding during the subsequent sintering phase.
Consequences of Skipping the Drying Phase
Gasification and Pore Formation
If solvents or moisture remain in the mold during vacuum hot press sintering, they will instantly gasify when temperatures rise. Because the mold is under high pressure (e.g., 30 MPa), this trapped gas has nowhere to escape. The resulting expansion creates internal pressure that forces the material apart, resulting in pore defects and voids within the composite.
Reduced Material Density
The primary goal of hot pressing is densification. Trapped gases act as a barrier between particles, preventing them from fusing completely. Skipping the drying phase inevitably leads to a final product with low density and compromised mechanical strength.
The Role of the Graphite Mold
Uniform Heat and Pressure Transfer
Graphite molds are selected for their ability to withstand immense pressure and transmit heat uniformly. However, because they act as a tightly sealed container under pressure, they can trap volatiles effectively. Drying the powder within the graphite mold ensures that the entire assembly is stabilized before the heavy hydraulic rams apply force.
Understanding the Trade-offs
Process Time vs. Material Quality
The primary trade-off of this step is an increase in total processing time. Adding a vacuum drying cycle requires additional equipment and extends the production timeline. However, this time cost is negligible compared to the cost of scrapping a sintered part due to porosity or cracking.
Handling and Logistics
Moving loaded graphite molds between a drying oven and a hot press requires careful handling. While graphite is strong at high temperatures, it can be brittle and susceptible to oxidation if handled improperly in air. Operators must minimize exposure to air between the drying and sintering stages to maintain the benefits of the vacuum treatment.
Making the Right Choice for Your Process
If you are optimizing your sintering workflow, consider the following regarding vacuum drying:
- If your primary focus is Maximum Density: Ensure the vacuum drying cycle is long enough to remove all adsorbed moisture, as this is the leading cause of microporosity.
- If your primary focus is Chemical Purity: Prioritize high-vacuum levels during drying to prevent the oxidation of sensitive metal alloy powders before sintering begins.
Ultimately, the vacuum drying stage is not merely a drying step; it is the fundamental defense against structural failure in high-performance composites.
Summary Table:
| Step | Purpose | Key Benefit |
|---|---|---|
| Vacuum Drying | Removes solvents (e.g., ethanol) and adsorbed moisture from powders in the mold. | Prevents gasification and pore formation during sintering. |
| Vacuum Hot Press Sintering | Applies high heat and pressure to densify the powder mixture. | Achieves final component density and mechanical strength. |
| Consequence of Skipping Drying | Trapped volatiles expand under heat and pressure. | Leads to voids, low density, and compromised material integrity. |
Achieve flawless material densification and avoid costly defects. The vacuum drying process is essential for high-performance composites. Backed by expert R&D and manufacturing, KINTEK offers Vacuum and other lab high-temp furnaces, all customizable for unique needs. Let our experts help you optimize your sintering workflow. Contact us today to discuss your application!
Visual Guide
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering and Brazing Furnace
People Also Ask
- What role does SPS equipment play in half-Heusler fabrication? Mastering Density and Microstructure for Thermoelectrics
- Why is Spark Plasma Sintering (SPS) preferred for Ba0.95La0.05FeO3-δ ceramics? Achieve High Density Fast
- What key role does a vacuum hot pressing furnace play in ADSC alloys? Achieve Near-Theoretical Density & Purity
- What are the main components of a vacuum hot press sintering furnace? Unlock Precision in Material Densification
- What are the primary technical advantages of using a Spark Plasma Sintering (SPS) system? Achieve Superior Sintering
- Which process parameters must be optimized for specific materials in a vacuum hot press furnace? Achieve Optimal Density and Microstructure
- How does a vacuum hot press machine improve material properties? Achieve Superior Strength and Purity
- How does vacuum hot press pressure influence Al-Si/graphite microstructure? Master Anisotropic Alignment



















