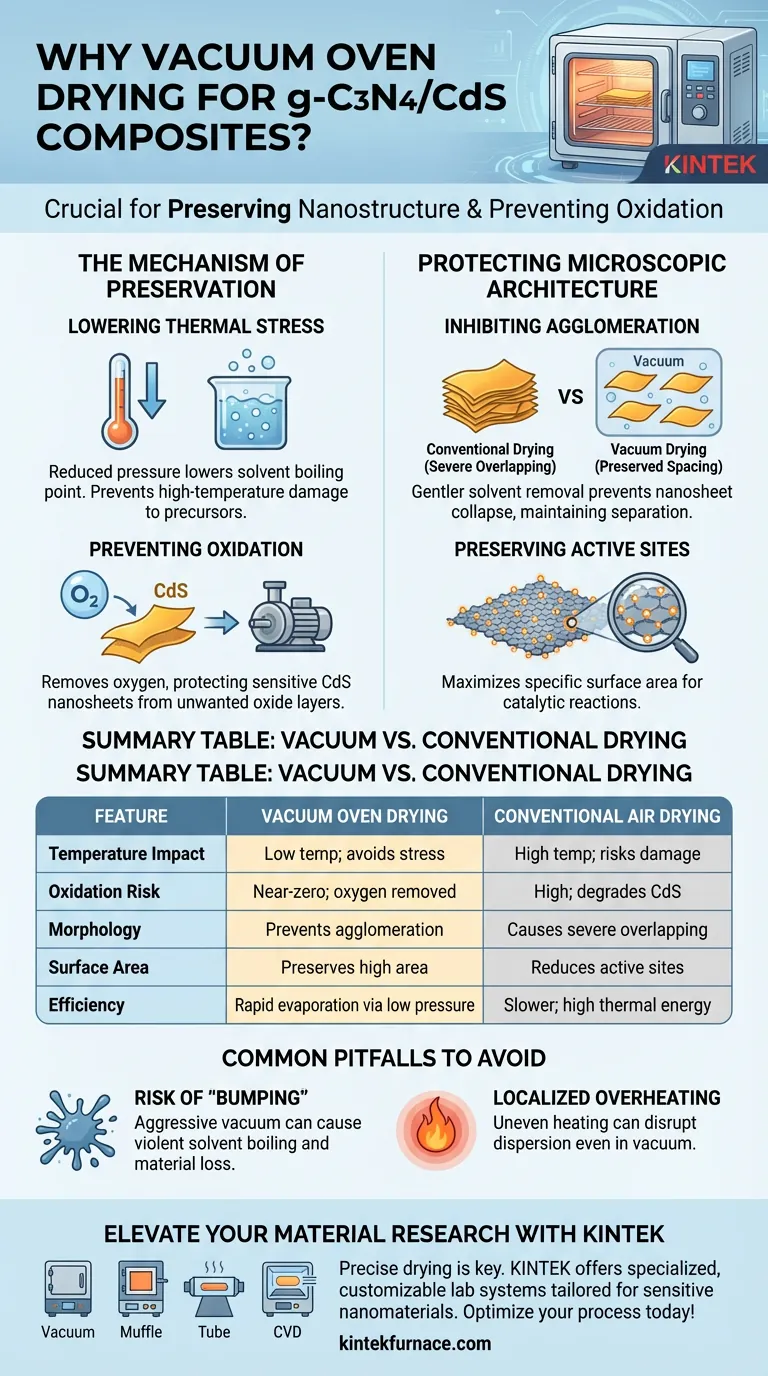
The primary reason for utilizing a vacuum oven in this process is to lower the boiling point of solvents, allowing the precursor materials to dry thoroughly at significantly reduced temperatures. For g-C3N4/CdS composites, this environment is critical to prevent the oxidation of sensitive Cadmium Sulfide (CdS) nanosheets and to stop them from severely overlapping or agglomerating, which would otherwise degrade the material's performance.
Core Takeaway Vacuum drying is not just about removing moisture; it is a preservation technique for nanostructures. By operating at reduced pressures, you protect the high specific surface area and chemical active sites of the CdS nanosheets from the structural collapse and oxidation associated with high-temperature air drying.
The Mechanism of Preservation
Lowering Thermal Stress
The fundamental advantage of a vacuum oven is its ability to reduce ambient pressure. This drop in pressure significantly lowers the boiling point of solvents like ethanol or water.
Consequently, solvents can be evaporated rapidly without subjecting the material to high temperatures. This is vital for maintaining the structural integrity of thermally sensitive precursors.
Preventing Oxidation
In a standard drying oven, high temperatures combined with ambient air can lead to rapid oxidation. This is particularly detrimental to ultrathin CdS nanosheets.
The vacuum environment effectively removes oxygen from the chamber. This ensures the chemical stability of the material is maintained throughout the drying phase, preventing the formation of unwanted oxide layers on the composite surface.
Protecting Microscopic Architecture
Inhibiting Agglomeration and Stacking
One of the greatest risks during the drying of 2D nanomaterials is the tendency for sheets to restack or clump together. High temperatures often exacerbate this "severe overlapping" and agglomeration.
Vacuum drying mitigates this by allowing for a gentler removal of solvents. This prevents the physical collapse of the nanosheets, preserving the spacing and separation required for a high-quality composite.
Preserving Active Sites
The performance of a g-C3N4/CdS composite relies heavily on its specific surface area. The more surface area available, the more "active sites" exist for catalytic reactions.
By preventing agglomeration and oxidation, the vacuum process preserves these active sites. It ensures the distinct 2D morphology of the CdS nanosheets remains intact for the subsequent compositing steps.
Common Pitfalls to Avoid
Risk of "Bumping" or Material Loss
While vacuum drying is efficient, applying vacuum too aggressively can cause solvents to boil violently (bump). This can displace the powder or cause it to splatter within the chamber.
Localized Overheating
Although the general temperature is lower, uneven heating can still occur if the equipment is not calibrated. Localized overheating can cause migration or pre-aggregation of active components, disrupting the metal dispersion even in a vacuum.
Making the Right Choice for Your Goal
To maximize the quality of your g-C3N4/CdS composite, consider your specific priorities:
- If your primary focus is maximzing catalytic efficiency: Prioritize the vacuum setting to prevent nanosheet overlapping, as this directly conserves the specific surface area and active sites.
- If your primary focus is chemical purity: Ensure the vacuum seal is robust to eliminate oxygen exposure, preventing secondary oxidation of the CdS surface.
Ultimately, the vacuum oven is the safeguard that ensures your precursor's delicate nano-architecture survives the transition from liquid slurry to solid composite.
Summary Table:
| Feature | Vacuum Oven Drying | Conventional Air Drying |
|---|---|---|
| Temperature Impact | Operates at low temp; avoids thermal stress | High temp required; risks structural damage |
| Oxidation Risk | Near-zero due to oxygen removal | High; risks degrading CdS nanosheets |
| Morphology | Prevents nanosheet agglomeration/stacking | Causes severe overlapping & clumping |
| Surface Area | Preserves high specific surface area | Reduces active sites for catalysis |
| Efficiency | Rapid solvent evaporation via low pressure | Slower; dependent on high thermal energy |
Elevate Your Material Research with KINTEK
Precise drying is the difference between a collapsed structure and a high-performance composite. Backed by expert R&D and manufacturing, KINTEK offers specialized Vacuum, Muffle, Tube, and CVD systems tailored for sensitive nanomaterials like g-C3N4/CdS. Our lab high-temp furnaces are fully customizable to prevent oxidation and preserve your active sites.
Ready to optimize your drying process? Contact us today to find the perfect thermal solution!
Visual Guide
References
- Muhammad Saad, Mazloom Shah. Development of stable S-scheme 2D–2D g-C3N4/CdS nanoheterojunction arrays for enhanced visible light photomineralisation of nitrophenol priority water pollutants. DOI: 10.1038/s41598-024-52950-3
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering and Brazing Furnace
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
People Also Ask
- How do the drying conditions in a vacuum drying oven affect NiS2 electrode quality? Optimize Your Battery Performance
- What is vacuum heat treatment? Achieve Superior Material Performance and Pristine Surfaces
- What is the primary role of a vacuum melting furnace in Ti-Zr-Mo-W alloy prep? Ensure Purity and Homogeneity
- What are some common applications of vacuum chamber furnaces? Unlock High-Purity Material Transformations
- What is the purpose of vacuum sintering furnaces? Achieve High-Purity, Dense Materials
- What processing conditions does a vacuum furnace provide for TiCp/Fe microspheres? Sintering at 900 °C
- What is the significance of temperature control in the condenser during the magnesium distillation process?
- What are the benefits of using a vacuum furnace for heat treatment? Achieve Clean, Precise Results for Your Materials



















