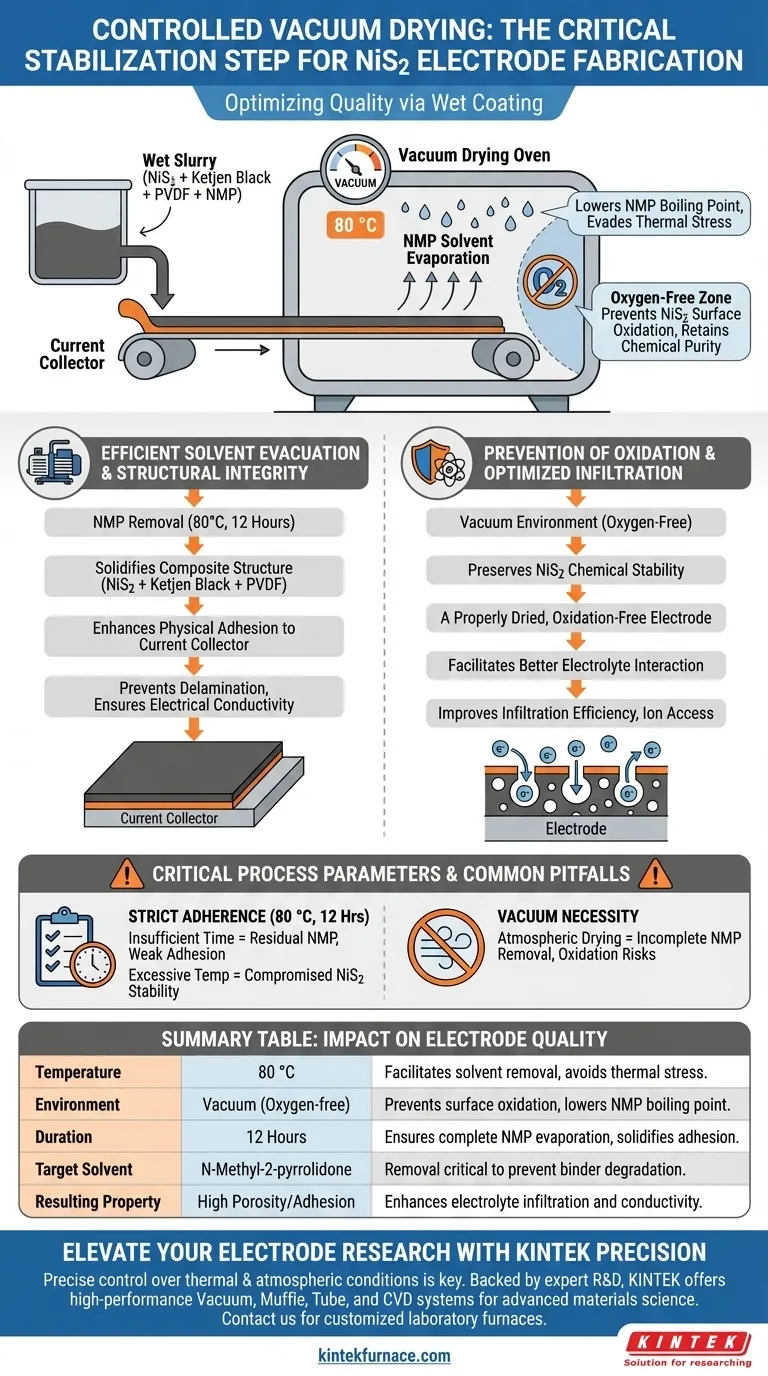
Controlled vacuum drying is the critical stabilization step in NiS2 electrode fabrication. It ensures electrode quality by simultaneously removing the N-Methyl-2-pyrrolidone (NMP) solvent and preventing material degradation. Specifically, maintaining a vacuum at 80 °C for 12 hours solidifies the physical contact between the electrode paste and the current collector while protecting the active material from oxidation.
The vacuum environment serves a dual purpose: it facilitates the complete removal of NMP solvent without requiring excessive heat, and it creates an oxygen-free zone to prevent the surface oxidation of Nickel Disulfide. This combination safeguards the material's structural integrity and enhances subsequent electrochemical performance.

The Role of Environmental Control
Efficient Solvent Evacuation
The primary mechanical goal of the drying process is the removal of the solvent, N-Methyl-2-pyrrolidone (NMP).
The vacuum environment lowers the boiling point of the solvent.
This allows the NMP to evaporate effectively at a moderate temperature of 80 °C over a 12-hour period, avoiding the thermal stress associated with higher temperatures.
Prevention of Surface Oxidation
Nickel Disulfide (NiS2) is susceptible to chemical changes when exposed to high temperatures in the presence of air.
Standard thermal drying could lead to surface oxidation of the active material.
By utilizing a vacuum oven, you eliminate oxygen from the drying chamber. This ensures the NiS2 retains its chemical purity and electrochemical properties during the heating phase.
Impact on Structural Integrity
Enhancing Physical Adhesion
The removal of the solvent solidifies the composite structure of the active material, Ketjen Black, and PVDF binder.
Deep drying ensures stable physical contact between this electrode layer and the current collector.
This strong adhesion is vital for maintaining electrical conductivity and preventing delamination during battery cycling.
Optimizing Electrolyte Infiltration
The quality of the drying process directly influences the electrode's porosity and surface condition.
A properly dried electrode, free from oxidation byproducts, facilitates better interaction with the electrolyte.
This improves electrolyte infiltration efficiency, ensuring ions can access the active material effectively.
Critical Process Parameters
Strict Adherence to Time and Temperature
The specified parameters of 80 °C for 12 hours are not arbitrary.
Insufficient time may leave residual NMP, which can degrade the binder's performance and weaken electrode adhesion.
Conversely, excessive temperatures without a vacuum could compromise the chemical stability of the NiS2.
The Necessity of the Vacuum
Attempting to achieve similar drying results without a vacuum is a common pitfall.
Atmospheric drying often fails to remove NMP completely at 80 °C.
Furthermore, it exposes the active material to oxidation risks that the vacuum environment specifically mitigates.
Optimizing Your Fabrication Process
To ensure high-performance NiS2 electrodes, you must view the drying phase as a chemical preservation step, not just a physical drying step.
- If your primary focus is Chemical Purity: Prioritize the integrity of the vacuum seal to ensure zero oxygen exposure, preventing surface oxidation of the active NiS2.
- If your primary focus is Mechanical Stability: Ensure the full 12-hour duration is met to guarantee complete solvent removal and robust adhesion to the current collector.
Proper vacuum drying transforms a wet slurry into a chemically stable, physically robust electrode ready for cell assembly.
Summary Table:
| Parameter | Specification | Impact on NiS2 Electrode Quality |
|---|---|---|
| Temperature | 80 °C | Facilitates solvent removal while avoiding thermal stress. |
| Environment | Vacuum (Oxygen-free) | Prevents surface oxidation of NiS2 and lowers NMP boiling point. |
| Duration | 12 Hours | Ensures complete NMP evaporation and solidifies binder adhesion. |
| Target Solvent | N-Methyl-2-pyrrolidone | Removal is critical to prevent binder degradation and delamination. |
| Resulting Property | High Porosity/Adhesion | Enhances electrolyte infiltration and electrical conductivity. |
Elevate Your Electrode Research with KINTEK Precision
Precise control over thermal and atmospheric conditions is the difference between a high-performance battery and a failing cell. Backed by expert R&D and manufacturing, KINTEK offers high-performance Vacuum, Muffle, Tube, and CVD systems tailored for advanced materials science.
Whether you are refining Nickel Disulfide electrodes or developing next-gen energy storage, our customizable laboratory furnaces provide the stability and oxygen-free environments your research demands.
Ready to optimize your fabrication process? Contact KINTEK today for a customized solution.
Visual Guide
References
- Milan K. Sadan, Hyo‐Jun Ahn. Overcoming copper-induced conversion reactions in nickel disulphide anodes for sodium-ion batteries. DOI: 10.1039/d3na00930k
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- Vacuum Induction Melting Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
People Also Ask
- How does a vacuum sintering furnace facilitate the final densification of 3D-printed fused silica glass? Expert Guide
- What are the advantages of vacuum brazing? Achieve Clean, Strong, and Stress-Free Joints
- In what common configurations are graphite heating elements arranged in vacuum furnaces? Optimize for Uniform Heating
- What energy-saving and environmental benefits do vacuum sintering furnaces offer? Boost Efficiency and Cut Emissions
- What is the function of a vacuum system in the vacuum distillation recovery process for magnesium alloys?
- How can manufacturers select the appropriate sintering furnace for their needs? Optimize Your Production with the Right Equipment
- What are the advantages of using a vacuum brazing furnace? Achieve Clean, Strong, and Flux-Free Joints
- How are parts cooled in vacuum carburizing, and what are the advantages? Achieve Superior Heat Treatment with Minimal Distortion



















